Soluble Semaphorin 4D ELISA | BI-20405
-
Method
Sandwich ELISA, HRP/TMB, 12×8-well detachable strips
-
Sample type
EDTA plasma, citrate plasma, heparın plasma, cell culture supernatant
-
Sample volume
10 µl / well
-
Assay time
3 h / 1 h / 30 min
-
Sensitivity
12 pmol/l (= 0.94 ng/ml)
-
Precision
In-between-run (n=11): ≤ 11 % CV
Within-run (n=5): ≤ 8 % CV
-
Standard range
0 – 2 000 pmol/l (= 0 – 157.8 ng/ml)
-
Conversion factor
1 pmol/l = 78.9 pg/ml (MW: 78.9 kDa)
-
Specificity
Endogenous and recombinant human soluble Semaphorin 4D.
-
Use
Research use only
-
Validation Data
See validation data tab for: precision, accuracy, dilution linearity, values for healthy donors, etc
Semaphorin 4D ELISA Product Overview
The Semaphorin 4D ELISA kit is a 4.5 hour, 96-well sandwich ELISA for the quantitative determination of soluble Sempahorin 4D (SEMA4D) in EDTA plasma, citrate plasma, heparın plasma and cell culture supernatant. The assay employs human plasma-based standards to ensure the measurement of biologically reliable data.
The soluble SEMA4D ELISA kit uses highly purified, epitope mapped antibodies. The antibodies utilized in the soluble Semaphorin-4D ELISA kit bind to AA30-AA34 and AA238-AA214 of soluble Semaphorin 4D.
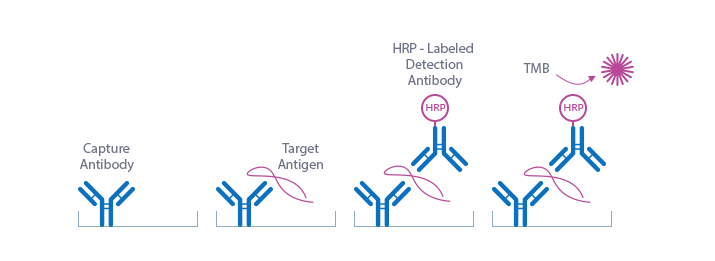
Semaphorin 4D ELISA Assay Principle
This Smephorin 4D ELISA kit is a sandwich enzyme immunoassay for the quantitative determination of soluble Semaphorin 4D in human plasma and cell culture supernatant.
The figure below explains the principle of the soluble Semaphorin-4D sandwich ELISA:
Capture antibody: monoclonal mouse anti-human Semaphorin 4D
Detection antibody: bivalent Fab bacterial alkaline phosphatase fusion antibody-HRP
Target antigen: human soluble Semaphorin 4D
In a first step, standards/controls/samples are pipetted into the wells of the microtiter strips, which are pre-coated with monoclonal mouse anti-human Semaphorin 4D antibody. Semaphorin 4D present in the standard/control/sample binds to the pre-coated antibody in the well. In a washing step, all non-specific unbound material is removed. In a next step, the conjugate (bivalent Fab bacterial alkaline phosphatase fusion antibody-HRP) is pipetted into the wells and reacts with the soluble Semaphorin 4D thereby forming a sandwich. After another washing step, the substrate (tetramethylbenzidine, TMB) is pipetted into the wells. The enzyme-catalyzed color change of the substrate is directly proportional to the amount of Semaphorin 4D present in the sample. This color change is detectable with a standard microtiter plate reader. A dose response curve of the absorbance (optical density, OD at 450 nm) vs. the standard concentration is generated. The concentration of Semaphorin 4D in the sample is determined directly from the dose response curve.
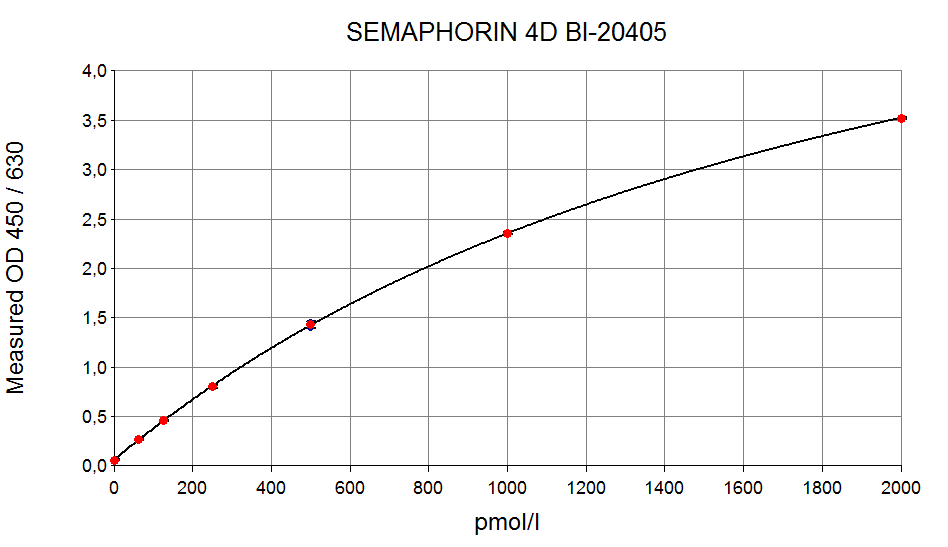
Semaphorin 4D ELISA Typical Standard Curve
The figure below shows a typical standard curve for the soluble SEMA4D ELISA. The immunoassay is calibrated against recombinant human Semaphorin 4D:
Semaphorin 4D ELISA Kit Components
|
Contents |
Description |
Quantity |
|
PLATE |
Monoclonal mouse anti-human Semaphorin 4D antibody pre-coated microtiter strips in a strip holder, packed in an aluminum bag with desiccant |
12 x 8 tests |
|
WASHBUF |
Wash buffer concentrate 20x, natural cap |
1 x 50 ml |
|
STD |
Standards 1-7, (0; 62.5; 125; 250; 500; 1,000; 2,000 pmol/l), recombinant human soluble Semaphorin 4D in human plasma, white caps, lyophilized |
7 vials |
|
CTRL |
Control A and B, yellow caps, lyophilized, exact concentrations see labels |
2 vials |
|
ASYBUF |
Assay buffer, red cap, ready to use |
1 x 13 ml |
|
CONJ |
Conjugate (bivalent Fab bacterial alkaline phosphatase fusion antibody-HRP), amber bottle, amber cap, ready to use |
1 x 13 ml |
|
SUB |
Substrate (TMB solution), blue cap, ready to use |
1 x 13 ml |
|
STOP |
STOP solution, white cap, ready to use |
1 x 7 ml |
Storage instructions: All reagents of the soluble Semaphorin 4D ELISA kit are stable at 4°C until the expiry date stated on the label of each reagent.
EDTA plasma, heparın plasma, citrate plasma and cell culture supernatant are suitable for use in this Semaphorin 4D ELISA. Do not change sample type during studies. We recommend duplicate measurements for all samples, standards and controls. The sample collection and storage conditions listed are intended as general guidelines.
Plasma
Collect venous blood samples in standardized blood collection tubes using EDTA, heparın or citrate as an anticoagulant. Perform separation by centrifugation according to the tube manufacturer’s instructions for use. Assay the acquired samples immediately or aliquot and store at -25°C or lower. Lipemic or haemolyzed samples may give erroneous results. Do not freeze-thaw samples more than four times.
Cell Culture Supernatant
Note: the experiments performed to measure soluble Semaphorin 4D cell culture supernatant samples did not undergo a full validation according to FDA/ICH/EMEA guidelines. However, our performance check suggests that cell culture supernatant samples can be measured with this ELISA.
Remove particulates by centrifugation and assay immediately or aliquot and store samples at -25°C or lower.
Reagent Preparation
Wash Buffer
|
1. |
Bring the WASHBUF concentrate to room temperature. Crystals in the buffer concentrate will dissolve at room temperature. |
|
2. |
Dilute the WASHBUF concentrate 1:20, e.g. 50 ml WASHBUF + 950 ml distilled or deionized water. Only use diluted WASHBUF when performing the assay. |
The diluted WASHBUF is stable up to one month at 4°C (2-8°C).
Standards for Plasma Measurements
|
1. |
Pipette 200 µl of distilled or deionized water into each standard (STD) and control (CTRL) vial. The exact concentration is printed on the label of each vial. |
|
2. |
Leave at room temperature (18-26°C) for 15 min. Vortex gently. Make sure that the lyophilisate is completely dissolved. |
Reconstituted STDs and the CTRL are stable at -25°C or lower until expiry date stated on the label. STDs and CTRLs are stable for four freeze-thaw cycles.
Standards for Cell Culture Supernatant Measurements
For the preparation of a cell culture-based standard curve always use the identical cell culture medium (CCM) as used for the experiment.
|
1. |
Reconstitute standard 7 (STD7) in 200 µl deionized water. Leave at room temperature (18-26°C) for 15 min and mix well before making dilutions. Use polypropylene tubes. |
|
2. |
Mark tubes ccSTD6 to ccSTD1. Dispense 50 µl cell culture medium into each vial. |
|
3. |
Pipette 50 µl of STD7 into tube marked as ccSTD6. Mix thoroughly. |
|
4. |
Transfer 50 µl of ccSTD6 into the tube marked as ccSTD5. Mix thoroughly. |
|
5. |
Continue in the same fashion to obtain ccSTD4 to ccSTD2. CCM serves as the ccSTD1 (0 pmol/l soluble Semaphorin 4D). |
|
6. |
Using the prepared standards, follow the protocol as indicated for plasma samples. |
Attention: Supplied STD1-STD7 and controls are only valid for plasma and should not be used for cell culture measurements.
Sample Preparation
Bring samples to room temperature and mix samples gently to ensure the samples are homogenous. We recommend duplicate measurements for all samples. Samples for which the optical density (OD) value exceeds the highest point of the standard range (STD7, 2 000 pmol/l) can be diluted with ASYBUF (assay buffer).
Semaphorin 4D ELISA Assay Protocol
Read the entire protocol before beginning the assay.
|
1. |
Bring reagents and samples to room temperature (18-26°C). |
|
2. |
Mark position for STD/CTRL/SAMPLE (standard/control/sample) on the protocol sheet. |
|
3. |
Take microtiter strips out of the aluminum bag. Store unused strips with desiccant at 4°C in the aluminum bag. Strips are stable until expiry date stated on the label. |
|
4. |
Pipette 100 µl ASYBUF (assay buffer, red cap) into each well. |
|
5. |
Add 10 µl STD/CTRL/SAMPLE in duplicates into the respective wells, swirl gently. |
|
6. |
Cover the plate tightly, swirl gently and incubate for 3 hours at room temperature (18-26°C). |
|
7. |
Aspirate and wash wells 5x with 300 µl diluted WASHBUF (wash buffer). After the final wash, remove the remaining WASHBUF by strongly tapping plate against a paper towel. |
|
8. |
Add 100 µl CONJ (conjugate, amber cap) into each well, swirl gently. |
|
9. |
Cover tightly and incubate for 1 hour at room temperature in the dark. |
|
10. |
Aspirate and wash wells 5x with 300 µl diluted WASHBUF. After the final wash, remove remaining WASHBUF by strongly tapping plate against a paper towel. |
|
11. |
Add 100 µl SUB (substrate, blue cap) into each well. |
|
12. |
Incubate for 30 min at room temperature in the dark. |
|
13. |
Add 50 µl STOP (stop solution, white cap) into each well. |
|
14. |
Measure absorbance immediately at 450 nm with a reference wavelength at 630 nm, if available. |
Calculation of Results
Read the optical density (OD) of all wells on a plate reader using 450 nm wavelength (reference wavelength 630 nm). Construct a standard curve from the absorbance read-outs of the standards using commercially available software capable of generating a four-parameter logistic (4-PL) fit. Alternatively, plot the standards’ concentration on the x-axis against the mean absorbance for each standard on the y-axis and draw a best fit curve through the points on the graph. Curve fitting algorithms other than 4-PL have not been validated and will need to be evaluated by the user.
Obtain sample concentrations from the standard curve. If required, pmol/l can be converted into pg/ml by applying a conversion factor (1 pg/ml = 0.00127 pmol/l (MW: 78.9 kDa)). Respective dilution factors have to be considered when calculating the final concentration of the sample.
The quality control (QC) protocol supplied with the kit shows the results of the final release QC for each kit lot. Data for OD obtained by customers may differ due to various influences and/or due to the normal decrease of signal intensity during shelf life. However, this does not affect validity of results as long as an OD of 1.50 or more is obtained for STD7 and the values of the CTRLs are in range (target ranges see labels).
Semaphorin 4D Protein
Semaphorin 4D (Sema4D, CD100) is a member of a family of transmembrane and secreted proteins that regulates key cellular functions and is involved in cell-cell communication (Kruger et al., 2005; Nkyimbeng-Takwi and Chapoval, 2011; Yazdani and Terman, 2006). Most of the effects of Semaphorin 4D are mediated by plexins (Janssen et al., 2012; Suzuki et al., 2008). Semaphorin 4D participates in numerous physiological processes such as axon guidance, immune regulation, angiogenesis, tumor progression, and bone metabolism (Conrotto et al., 2005; Negishi-Koga et al., 2011a; Negishi-Koga and Takayanagi, 2012; Takamatsu and Kumanogoh, 2012). Cleavage of Semaphorin 4D near the cell membrane through matrix metalloproteinases leads to the biologically active soluble Semaphorin 4D with a molecular weight of 120 kD consisting of 713 amino acids (Maleki et al., 2016; Nkyimbeng-Takwi and Chapoval, 2011; Suzuki et al., 2008). Semaphorin 4D has emerged as a novel therapeutic target in cancer and in bone diseases (Fisher et al., 2016; Yufeng Zhang et al., 2015). Semaphorin 4D is widely studied for its role in neural connectivity, vascularization, cell migration, immune responses, tumor progression, and bone remodeling.
|
Molecular weight |
78.9 kDa |
|
Cellular localisation |
Cell membrane, extracellular |
|
Post-translational modifications |
Glycosylation |
|
Sequence similarities |
Semaphorins |
|
Alternative names |
Semaphorin 4D, Semaphorin-4D, C9orf164, SEMAJ, CD100, BB18, GR3, CD100 Antigen, M-Sema-G, M-Sema G, Coll-4, COLL4 |
|
Entrez/NCBI ID |
|
|
Genecards |
|
|
OMIM |
|
|
PDB |
|
|
Pfam |
|
|
Protein Atlas |
|
|
Uniprot ID |
Semaphorin 4D Function
Semaphorin 4D (Sema4D, CD100) is a type I integral membrane glycoprotein expressed as a disulphide-linked homodimer. It is over-expressed in a wide variety of cancers including malignancies of prostate, colon, breast, lung, and pancreas, as well as cervical and ovarian malignancies, head and neck squamous cell carcinoma, and osteosarcoma (Basile et al., 2006; Campos et al., 2013; Kato et al., 2011; Liu et al., 2014; Moriarity et al., 2015).
The extracellular region of Semaphorin 4D can be proteolytically cleaved to generate a soluble molecule retaining its biological activity (Basile et al., 2007). The type 1 matrix metalloproteinases mediating this cleavage are upregulated in many malignant cells (Arribas and Esselens, 2009; Strongin, 2010). Among the three receptors binding soluble and transmembrane Semaphorin 4D, Plexin B1 has the highest affinity and is expressed on antigen presenting cells, endothelial and epithelial cells, as well as on some cancer cells (Ch’ng and Kumanogoh, 2010; Conrotto, 2005; Tamagnone et al., 1999).
Semaphorin 4D activates endothelial cells and promotes tumor angiogenesis and tumor progression (Basile et al., 2006, 2004; Conrotto, 2005). Furthermore, it influences vascular permeability and might thereby regulate extravasation (Zhou et al., 2014). Apart from its pro-angiogenic properties, Semaphorin 4D acts on receptor-positive malignant cells where it promotes survival, proliferation, and migration (Capparuccia and Tamagnone, 2009; Chen et al., 2018; Damola et al., 2013; Takada et al., 2017). Within the tumor microenvironment, Semaphorin 4D influences the infiltration and differentiation of immune cells creating an anti-inflammatory milieu (Chen et al., 2013; Delaire et al., 2001; Evans et al., 2015). Moreover, Semaphorin 4D suppresses osteoblast differentiation and, hence, promotes the formation of bone metastasis (Negishi-Koga et al., 2011b; Takada et al., 2017; Yang et al., 2016a).
Elevated expression of Semaphorin 4D is generally associated with a poor prognosis in several malignancies (Chen et al., 2012, 2013; Kato et al., 2011; Liu et al., 2014; Wang et al., 2015). However, as a therapeutic target, interferences with Semaphorin 4D signaling provides the possibility to enhance anti-tumor immune responses and inhibit tumor progression (Evans et al., 2015; Patnaik et al., 2016). Recently, high expression of soluble Semaphorin 4D in the plasma of patients with head and neck squamous cell carcinoma has been reported (Derakhshandeh et al., 2018). This finding indicates that determination of Semaphorin 4D plasma levels might be useful tool to further study the role of Semaphorin 4D in the context of cancer progression, prognosis, and therapy.
-
Cardiology
Atrial fibrillation (Xiang et al., 2015)
Heart failure (Lu et al., 2013)
-
Immunology
Systemic sclerosis (Besliu et al., 2011)
Rheumatoid arthritis (Ha et al., 2018; Yoshida et al., 2015)
-
Oncology
Breast cancer (Jiang et al., 2016; Malik et al., 2015; Yang et al., 2016b)
Cervical cancer (Liu et al., 2014)
Colorectal cancer (Ding et al., 2016; Ikeya et al., 2016; Wang et al., 2015)
Epithelial ovarian cancer (Chen et al., 2018, 2013, 2012)
Gastric cancer (Li et al., 2018)
Head and neck cancer (Derakhshandeh et al., 2018)
Lung cancer (Chen et al., 2019)
Multiple myeloma (Terpos et al., 2018)
Oral squamous cancer (Zhou et al., 2017)
Pancreatic cancer (Kato et al., 2011)
Prostate cancer (Damola et al., 2013)
Soft tissue sarcomas (Campos et al., 2013)
Literature
Chen, W.-G., Sun, J., Shen, W.-W., Yang, S.-Z., Zhang, Y., Hu, X., Qiu, H., Xu, S.-C., Chu, T.-W., 2019. Clin. Exp. Metastasis.

Chen, Y., Zhang, L., Liu, W., Wang, K., 2018. Cell. Mol. Biol. Lett. 23.
Derakhshandeh, R., Sanadhya, S., Han, K.L., Chen, H., Goloubeva, O., Webb, T.J., Younis, R.H., 2018. Oncotarget 9.
Ha, Y.-J., Han, D.W., Kim, J.H., Chung, S.W., Kang, E.H., Song, Y.W., Lee, Y.J., 2018. Dis. Markers 2018, 2318386.

Promotion of Sema4D expression by tumor-associated macrophages: Significance in gastric carcinoma.
Li, H., Wang, J.-S., Mu, L.-J., Shan, K.-S., Li, L.-P., Zhou, Y.-B., 2018. World J. Gastroenterol. 24, 593–601.

Terpos, E., Ntanasis-Stathopoulos, I., Christoulas, D., Bagratuni, T., Bakogeorgos, M., Gavriatopoulou, M., Eleutherakis-Papaiakovou, E., Kanellias, N., Kastritis, E., Dimopoulos, M.A., 2018. Blood Cancer J. 8, 42.

Semaphorin 4D promotes bone invasion in head and neck squamous cell carcinoma.
Takada, H., Ibaragi, S., Eguchi, T., Okui, T., Obata, K., Masui, M., Morisawa, A., Takabatake, K., Kawai, H., Yoshioka, N., Hassan, N.M.M., Shimo, T., Hu, G.-F., Nagatsuka, H., Sasaki, A., 2017. Int. J. Oncol. 51, 625–632.
Zhou, H., Kann, M.G., Mallory, E.K., Yang, Y.-H., Bugshan, A., Binmadi, N.O., Basile, J.R., 2017. Neoplasia N. Y. N 19, 65–74.

The role of semaphorin 4D as a potential biomarker for antiangiogenic therapy in colorectal cancer.
Ding, X., Qiu, L., Zhang, L., Xi, J., Li, D., Huang, X., Zhao, Y., Wang, X., Sun, Q., 2016. OncoTargets Ther. 9, 1189–1204.

Generation and preclinical characterization of an antibody specific for SEMA4D.
Fisher, T.L., Reilly, C.A., Winter, L.A., Pandina, T., Jonason, A., Scrivens, M., Balch, L., Bussler, H., Torno, S., Seils, J., Mueller, L., Huang, H., Klimatcheva, E., Howell, A., Kirk, R., Evans, E., Paris, M., Leonard, J.E., Smith, E.S., Zauderer, M., 2016. mAbs 8, 150–162.
Ikeya, T., Maeda, K., Nagahara, H., Shibutani, M., Iseki, Y., Hirakawa, K., 2016. BMC Cancer 16.

The role of semaphorin 4D in tumor development and angiogenesis in human breast cancer.
Jiang, H., Chen, C., Sun, Q., Wu, J., Qiu, L., Gao, C., Liu, W., Yang, J., Jun, N., Dong, J., 2016. OncoTargets Ther. 9, 5737–5750.

Soluble SEMA4D/CD100: A novel immunoregulator in infectious and inflammatory diseases.
Maleki, K.T., Cornillet, M., Björkström, N.K., 2016. Clin. Immunol. 163, 52–59.
Patnaik, A., Weiss, G.J., Leonard, J.E., Rasco, D.W., Sachdev, J.C., Fisher, T.L., Winter, L.A., Reilly, C., Parker, R.B., Mutz, D., Blaydorn, L., Tolcher, A.W., Zauderer, M., Ramanathan, R.K., 2016. Clin. Cancer Res. 22, 827–836.
Semaphorin 4D Promotes Skeletal Metastasis in Breast Cancer.
Yang, Y.-H., Buhamrah, A., Schneider, A., Lin, Y.-L., Zhou, H., Bugshan, A., Basile, J.R., 2016a. PLOS ONE 11, e0150151.
Semaphorin 4D Promotes Skeletal Metastasis in Breast Cancer.
Yang, Y.-H., Buhamrah, A., Schneider, A., Lin, Y.-L., Zhou, H., Bugshan, A., Basile, J.R., 2016b. PLOS ONE 11, e0150151.
Evans, E.E., Jonason, A.S., Bussler, H., Torno, S., Veeraraghavan, J., Reilly, C., Doherty, M.A., Seils, J., Winter, L.A., Mallow, C., Kirk, R., Howell, A., Giralico, S., Scrivens, M., Klimatcheva, K., Fisher, T.L., Bowers, W.J., Paris, M., Smith, E.S., Zauderer, M., 2015. Cancer Immunol. Res. 3, 689–701.
Malik, M.F.A., Ye, L., Jiang, W.G., 2015. Oncol. Rep. 34, 1049–1057.
Moriarity, B.S., Otto, G.M., Rahrmann, E.P., Rathe, S.K., Wolf, N.K., Weg, M.T., Manlove, L.A., LaRue, R.S., Temiz, N.A., Molyneux, S.D., Choi, K., Holly, K.J., Sarver, A.L., Scott, M.C., Forster, C.L., Modiano, J.F., Khanna, C., Hewitt, S.M., Khokha, R., Yang, Y., Gorlick, R., Dyer, M.A., Largaespada, D.A., 2015. Nat. Genet. 47, 615–624.
Wang, J.-S., Jing, C.-Q., Shan, K.-S., Chen, Y.-Z., Guo, X.-B., Cao, Z.-X., Mu, L.-J., Peng, L.-P., Zhou, M.-L., Li, L.-P., 2015. World J. Gastroenterol. 21, 2191–2198.
Serum Soluble Semaphorin 4D is Associated with Left Atrial Diameter in Patients with Atrial Fibrillation.
Xiang, L., You, T., Chen, J., Xu, W., Jiao, Y., 2015. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 21, 2912–2917.

Yoshida, Y., Ogata, A., Kang, S., Ebina, K., Shi, K., Nojima, S., Kimura, T., Ito, D., Morimoto, K., Nishide, M., Hosokawa, T., Hirano, T., Shima, Y., Narazaki, M., Tsuboi, H., Saeki, Y., Tomita, T., Tanaka, T., Kumanogoh, A., 2015. Arthritis Rheumatol. 67, 1481–1490.
Zhang, Yiyuan, Feng, E., Xu, Y., Wang, W., Zhang, T., Xiao, L., Chen, R., Lin, Yu, Chen, D., Lin, L., Chen, K., Lin, Yanbin, 2015. Int. J. Clin. Exp. Med. 8, 16352–16357

Zhang, Yufeng, Wei, L., Miron, R.J., Shi, B., Bian, Z., 2015. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 30, 286–296.

Semaphorin 4D expression is associated with a poor clinical outcome in cervical cancer patients.
Liu, H., Yang, Y., Xiao, J., Yang, S., Liu, Y., Kang, W., Li, X., Zhang, F., 2014. Microvasc. Res. 93, 1–8.
Zhou, H., Yang, Y.-H., Basile, J.R., 2014. Angiogenesis 17, 261–274.
Campos, M., De Campos, S.G.P., Ribeiro, G.G., Eguchi, F.C., Silva, S.R.M.D., De Oliveira, C.Z., Da Costa, A.M., Curcelli, E.C., Nunes, M.C., Penna, V., Longatto-Filho, A., 2013. Oncol. Lett. 5, 1527–1535.
Chen, Y., Zhang, L., Lv, R., Zhang, W.-Q., 2013. Asian Pac. J. Cancer Prev. 14, 5883–5890.
Function of mutant and wild-type plexinB1 in prostate cancer cells: PlexinB1 and Prostate Cancer.
Damola, A., Legendre, A., Ball, S., Masters, J.R., Williamson, M., 2013. The Prostate 73, 1326–1335.
Increased Levels of Plasma Soluble Sema4D in Patients with Heart Failure.
Lu, Q., Dong, N., Wang, Q., Yi, W., Wang, Y., Zhang, S., Gu, H., Zhao, X., Tang, X., Jin, B., Wu, Q., Brass, L.F., Zhu, L., 2013. PLoS ONE 8, e64265.
Chen, Y., Zhang, L., Pan, Y., Ren, X., Hao, Q., 2012. Int. J. Mol. Sci. 13, 13264–13274.
Neuropilins lock secreted semaphorins onto plexins in a ternary signaling complex.
Janssen, B.J.C., Malinauskas, T., Weir, G.A., Cader, M.Z., Siebold, C., Jones, E.Y., 2012. Nat. Struct. Mol. Biol. 19, 1293–1299.
Bone cell communication factors and Semaphorins.
Negishi-Koga, T., Takayanagi, H., 2012. BoneKEy Rep. 1, 183.

Diverse roles for semaphorin-plexin signaling in the immune system.
Takamatsu, H., Kumanogoh, A., 2012. Trends Immunol. 33, 127–135.

Besliu, A., Banica, L., Predeteanu, D., Vlad, V., Ionescu, R., Pistol, G., Opris, D., Berghea, F., Stefanescu, M., Matache, C., 2011. Autoimmunity 44, 427–436.
Kato, S., Kubota, K., Shimamura, T., Shinohara, Y., Kobayashi, N., Watanabe, S., Yoneda, M., Inamori, M., Nakamura, F., Ishiguro, H., Nakaigawa, N., Nagashima, Y., Taguri, M., Kubota, Y., Goshima, Y., Morita, S., Endo, I., Maeda, S., Nakajima, A., Nakagama, H., 2011. Cancer Sci. 102, 2029–2037.
Suppression of bone formation by osteoclastic expression of semaphorin 4D.
Negishi-Koga, T., Shinohara, M., Komatsu, N., Bito, H., Kodama, T., Friedel, R.H., Takayanagi, H., 2011a. Nat. Med. 17, 1473–1480.
Suppression of bone formation by osteoclastic expression of semaphorin 4D.
Negishi-Koga, T., Shinohara, M., Komatsu, N., Bito, H., Kodama, T., Friedel, R.H., Takayanagi, H., 2011b. Nat. Med. 17, 1473–1480.
Biology and function of neuroimmune semaphorins 4A and 4D.
Nkyimbeng-Takwi, E., Chapoval, S.P., 2011. Immunol. Res. 50, 10–21.

Roles of Sema4D and Plexin-B1 in tumor progression.
Ch’ng, E.S., Kumanogoh, A., 2010. Mol. Cancer 9, 251.
Proteolytic and non-proteolytic roles of membrane type-1 matrix metalloproteinase in malignancy.
Strongin, A.Y., 2010. Biochim. Biophys. Acta 1803, 133–141.
ADAM17 as a Therapeutic Target in Multiple Diseases.
Arribas, J., Esselens, C., 2009. Curr. Pharm. Des. 15, 2319–2335.
Capparuccia, L., Tamagnone, L., 2009. J. Cell Sci. 122, 1723–1736.
Semaphorins and their receptors in immune cell interactions.
Suzuki, K., Kumanogoh, A., Kikutani, H., 2008. Nat. Immunol. 9, 17–23.
MT1-MMP Controls Tumor-induced Angiogenesis through the Release of Semaphorin 4D.
Basile, J.R., Holmbeck, K., Bugge, T.H., Gutkind, J.S., 2007. J. Biol. Chem. 282, 6899–6905.
Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis.
Basile, J.R., Castilho, R.M., Williams, V.P., Gutkind, J.S., 2006. Proc. Natl. Acad. Sci. 103, 9017–9022.
Yazdani, U., Terman, J.R., 2006. Genome Biol. 7, 211.

Sema4D induces angiogenesis through Met recruitment by Plexin B1.
Conrotto, P., 2005. Blood 105, 4321–4329.
Sema4D induces angiogenesis through Met recruitment by Plexin B1.
Conrotto, P., Valdembri, D., Corso, S., Serini, G., Tamagnone, L., Comoglio, P.M., Bussolino, F., Giordano, S., 2005. Blood 105, 4321–4329.
Semaphorins command cells to move.
Kruger, R.P., Aurandt, J., Guan, K.-L., 2005. Nat. Rev. Mol. Cell Biol. 6, 789–800.
Class IV Semaphorins Promote Angiogenesis by Stimulating Rho-Initiated Pathways through Plexin-B.
Basile, J.R., Barac, A., Zhu, T., Guan, K.-L., Gutkind, J.S., 2004. Cancer Res. 64, 5212–5224.
Delaire, S., Billard, C., Tordjman, R., Chedotal, A., Elhabazi, A., Bensussan, A., Boumsell, L., 2001. J. Immunol. 166, 4348–4354.
Tamagnone, L., Artigiani, S., Chen, H., He, Z., Ming, G., Song, H., Chedotal, A., Winberg, M.L., Goodman, C.S., Poo, M., Tessier-Lavigne, M., Comoglio, P.M., 1999. Cell 99, 71-80.

All Biomedica ELISAs are validated according to international FDA/ICH/EMEA guidelines. For more information about our validation guidelines, please refer to our quality page and published validation guidelines and literature.
- ICH Q2(R1) Validation of Analytical Procedures: Text and Methodology
- EMEA/CHMP/EWP/192217/2009 Guideline on bioanalytical method validation
- Bioanalytical Method Validation, Guidance for Industry, FDA, May 2018
Calibration
The soluble Semaphorin 4D immunoassay is calibrated against recombinant soluble Semaphorin 4D protein (AA22-734 of Q92854 (Uniprot ID)).
Semaphorin 4D ELISA Detection Limit & Sensitivity
To determine the sensitivity of the soluble Semaphorin 4D ELISA, experiments measuring the lower limit of detection (LOD) and the lower limit of quantification (LLOQ) were conducted.
The LOD, also called the detection limit, is the lowest point at which a signal can be distinguished above the background signal, i.e. the signal that is measured in the absence of soluble Semaphorin 4D, with a confidence level of 99%. It is defined as the mean back calculated concentration of standard 1 (0 pmol/l of soluble Semaphorin 4D) plus three times the standard deviation of the measurements.
The LLOQ, or sensitivity of an assay, is the lowest concentration at which an analyte can be accurately quantified. The criteria for accurate quantification at the LLOQ are an analyte recovery between 75 and 125% and a coefficient of variation (CV) of less than 25%. To determine the LLOQ, standard 2, i.e. the lowest standards containing soluble Semaphorin 4D, is diluted, measured and its concentration back calculated. The lowest dilution, which meets both criteria, is reported as the LLOQ.
The following values were determined for the soluble Semaphorin 4D ELISA:
|
LOD |
12 pmol/l |
|
LLOQ |
31 pmol/l |
Semaphorin 4D ELISA Precision
The precision of an ELISA is defined as its ability to measure the same concentration consistently within the same experiments carried out by one operator (within-run precision or repeatability) and across several experiments using the same samples but conducted by several operators at different locations using different ELISA lots (in-between-run precision or reproducibility).
Within-Run Precision
Within-run precision was tested by measuring the same samples five times within one soluble Semaphorin 4D ELISA lot. The experiment was conducted by one operator.
|
ID |
n |
Mean sSEMA4D [pmol/l] |
SD [pmol/l] |
CV (%) |
|
Sample 1 |
5 |
126 |
10.4 |
8 |
|
Sample 2 |
5 |
1 003 |
63.8 |
6 |
In-Between-Run Precision
In-between-run precision was assessed by measuring the same samples eleven times within two soluble Semaphorin 4D ELISA lots. The measurements were carried out by four different operators.
|
ID |
n |
Mean sSEMA4D [pmol/l] |
SD [pmol/l] |
CV (%) |
|
Sample 1 |
11 |
134 |
14.4 |
11 |
|
Sample 2 |
11 |
1 012 |
55.1 |
5 |
Semaphorin 4D ELISA Accuracy
The accuracy of an ELISA is defined as the precision with which it can recover samples of known concentrations.
The recovery of the soluble Semaphorin 4D ELISA was measured by adding recombinant soluble Semaphorin 4D to human samples containing a known concentration endogenous soluble Semaphorin 4D. The % recovery of the spiked concentration was calculated as the percentage of measured compared over the expected value.
This table shows the summary of the recovery experiments in the soluble Semaphorin 4D ELISA in different sample matrices:
|
% Recovery |
|||||
|
Sample matrix |
n |
+200 pmol/l |
+1 000 pmol/l |
||
|
Mean |
Range |
Mean |
Range |
||
|
EDTA plasma |
6 |
116 |
102 – 136 |
92 |
79 - 104 |
|
Citrate plasma |
2 |
94 |
82 – 106 |
109 |
103 - 114 |
|
Heparın plasma |
2 |
79 |
78 - 80 |
83 |
82 - 83 |
|
sSEMA4D [pmol/l] |
% Recovery |
|||||
|
Sample matrix |
ID |
Ref |
+ 200 pmol/l |
+ 1 000 pmol/l |
+ 200 pmol/l |
+ 1 000 pmol/l |
|
EDTA plasma |
e1 |
323 | 527 | 1 367 | 102 | 104 |
|
EDTA plasma |
e2 |
244 | 480 | 1 032 | 118 | 79 |
|
EDTA plasma |
e3 |
337 | 608 | 1 208 | 136 | 87 |
|
EDTA plasma |
e4 |
378 | 634 | 1 360 | 128 | 98 |
|
EDTA plasma |
e5 |
413 | 626 | 1 322 | 106 | 91 |
|
EDTA plasma |
e6 |
261 | 469 | 1 175 | 104 | 91 |
|
Mean |
116 | 92 | ||||
|
|
|
|
|
Min |
102 | 79 |
|
|
|
|
|
Max |
136 | 104 |
Data showing recovery of recombinant soluble Semaphorin 4D in a human citrate plasma sample:
|
sSEMA4D [pmol/l] |
% Recovery |
|||||
|
Sample matrix |
ID |
Ref |
+ 200 pmol/l |
+ 1 000 pmol/l |
+ 200 pmol/l |
+ 1 000 pmol/l |
|
Citrate plasma |
c1 | 258 | 470 | 1 399 | 106 | 114 |
|
Citrate plasma |
c2 | 356 | 520 | 1 387 | 82 | 103 |
Data showing recovery of soluble Semaphorin 4D in human heparın plasma samples:
|
sSEMA4D [pmol/l] |
% Recovery |
|||||
|
Sample matrix |
ID |
Ref |
+ 200 pmol/l |
+ 1 000 pmol/l |
+ 200 pmol/l |
+ 1 000 pmol/l |
|
Heparın plasma |
h1 |
297 |
458 |
1 117 |
80 |
82 |
|
Heparın plasma |
h2 |
314 |
469 |
1 144 |
78 |
83 |
Semaphorin 4D ELISA Dilution Linearity & Parallelism
Tests of dilution linearity and parallelism ensure that both endogenous and recombinant samples containing soluble Semaphorin 4D behave in a dose dependent manner and are not affected by matrix effects. Dilution linearity assesses the accuracy of measurements in diluted human samples spiked with known concentrations of recombinant analyte. By contrast, parallelism refers to dilution linearity in human samples and provides evidence that the endogenous analyte behaves the same way as the recombinant one. Dilution linearity and parallelism are considered acceptable if the results are within ± 20% of the expected concentration.
Dilution Linearity
Dilution linearity was assessed by serially diluting human samples spiked with 1,000 pmol/l recombinant soluble Semaphorin 4D with standard 1 (human plasma, containing no soluble Semaphorin 4D).
The table below shows the mean recovery and range of serially diluted recombinant soluble Semaphorin 4D in EDTA plasma:
|
% Recovery of recombinant sSEMA4D in diluted samples |
|||||||||
|
1+1 |
1+3 |
1+7 |
1+15 |
||||||
|
Sample matrix |
n |
Mean |
Range |
Mean |
Range |
Mean |
Range |
Mean |
Range |
|
EDTA plasma |
6 |
103 | 87-118 | 106 | 83-124 | 82 | 56-102 | 94 | 80-114 |
Data showing dilution linearity of 1 000 pmol/l recombinant soluble Semaphorin 4D spiked into human EDTA plasma samples (reference) containing endogenous soluble Semaphorin 4D:
|
sSEMA4D [pmol/l] |
% Recovery |
|||||||||
|
Sample matrix |
ID |
Ref + 1000 pmol/l |
1+1 |
1+3 |
1+7 |
1+15 |
1+1 |
1+3 |
1+7 |
1+15 |
|
EDTA plasma |
e1 |
1,311 |
678 |
272 |
91 |
71 |
103 |
83 |
56 |
86 |
|
EDTA plasma |
e2 |
971 |
525 |
279 |
98 |
69 |
108 |
115 |
81 |
114 |
|
EDTA plasma |
e3 |
1,124 |
546 |
297 |
103 |
56 |
97 |
106 |
73 |
80 |
|
EDTA plasma |
e4 |
1,15 |
603 |
310 |
137 |
63 |
105 |
108 |
95 |
88 |
|
EDTA plasma |
e5 |
1,159 |
502 |
293 |
148 |
64 |
87 |
101 |
102 |
88 |
|
EDTA plasma |
e6 |
1,122 |
663 |
349 |
119 |
75 |
118 |
124 |
85 |
107 |
|
Mean |
103 |
106 |
82 |
94 |
||||||
|
Min |
87 |
83 |
56 |
80 |
||||||
|
Max |
118 |
124 |
102 |
114 |
||||||
Parallelism
Parallelism was assessed by serially diluting human samples containing endogenous soluble Semaphorin 4D with standard 1 (human plasma containing no soluble Semaphorin 4D).
The table below shows the mean recovery and range of serially diluted endogenous soluble Semaphorin 4D in several sample matrices:
|
% Recovery of endogenous sSEMA4D in diluted samples |
|||||||
|
Sample matrix |
n |
1+1 |
1+3 |
1+7 |
|||
|
Mean |
Range |
Mean |
Range |
Mean |
Range |
||
|
EDTA plasma |
4 |
106 |
93 – 126 |
92 |
89 – 106 |
99 |
90 - 105 |
|
Citrate plasma |
2 |
110 |
109 – 112 |
109 |
104 – 114 |
121 |
119 - 124 |
|
Heparın plasma |
2 |
103 |
98 – 107 |
93 |
77 – 110 |
133 |
89 - 177 |
Data showing dilution linearity of endogenous soluble Semaphorin 4D in human EDTA plasma samples:
|
sSEMA4D [pmol/l] |
% Recovery |
|||||||
|
Sample matrix |
ID |
Ref |
1+1 |
1+3 |
1+7 |
1+1 |
1+3 |
1+7 |
|
EDTA plasma |
e1 |
789 |
425 |
210 |
104 |
108 |
106 |
105 |
|
EDTA plasma |
e2 |
967 |
608 |
200 |
126 |
126 |
83 |
104 |
|
EDTA plasma |
e3 |
1 106 |
515 |
252 |
133 |
93 |
91 |
97 |
|
EDTA plasma |
e4 |
976 |
471 |
217 |
109 |
96 |
89 |
90 |
|
Mean |
106 |
92 |
99 |
|||||
|
Min |
93 |
89 |
90 |
|||||
|
Max |
108 |
106 |
105 |
|||||
Data showing dilution linearity of endogenous soluble Semaphorin 4D in human citrate plasma samples:
|
sSEMA4D [pmol/l] |
% Recovery |
|||||||
|
Sample matrix |
ID |
Ref |
1+1 |
1+3 |
1+7 |
1+1 |
1+3 |
1+7 |
|
Citrate plasma |
c1 |
274 |
153 |
78 |
41 |
112 |
114 |
119 |
|
Citrate plasma |
c2 |
272 |
148 |
70 |
42 |
109 |
104 |
124 |
Data showing dilution linearity of endogenous soluble Semaphorin 4D in a human heparın plasma sample:
|
sSEMA4D [pmol/l] |
% Recovery |
|||||||
|
Sample matrix |
ID |
Ref |
1+1 |
1+3 |
1+7 |
1+1 |
1+3 |
1+7 |
|
Heparın plasma |
h1 |
403 |
197 |
77 |
45 |
98 |
77 |
89 |
|
Heparın plasma |
h2 |
326 |
175 |
90 |
72 |
107 |
110 |
177 |
Semaphorin 4D ELISA Specificity
The specificity of an ELISA is defined as its ability to exclusively recognize an analyte, which means that the ELISA antibodies will bind to the target analyte while not binding to other molecules in solution.
To characterize the antibody pair, both the capture and the detection antibodies were characterized through epitope mapping and affinity measurements. In addition, the specificity of the ELISA was established through competition experiments, which measure the ability of the antibodies to exclusively bind soluble Semaphorin 4D.
Epitope Mapping
Antibody binding sites were determined by epitope mapping using microarray analysis (Pepperprint GmbH).
The monoclonal capture antibody was determined to bind to AA30-AA34.
The bivalent Fab bacterial alkaline phosphatase fusion antibody-HRP binds to region AA 238-241.
Isoforms
Isoform 1 and 2 are identical between AA 1-554. The epitopes of the antibodies utilized in this ELISA are situated in this area.
Competition of Signal
Competition experiments were carried out by pre-incubating human samples with a ten-fold excess of coating antibody. The concentration measured in this mixture was then compared to a reference value, which was obtained from the same sample but without the pre-incubation step. Mean competition was 98%.
|
sSEMA4D [pmol/l] |
% Competition |
|||
|
Sample matrix |
ID |
Ref |
Ref+ antibody |
|
|
EDTA plasma |
e1 |
926 |
3 |
100 |
|
EDTA plasma |
e2 |
761 |
3 |
100 |
|
EDTA plasma |
e3 |
790 |
3 |
100 |
|
EDTA plasma |
e4 |
323 |
0 |
100 |
|
EDTA plasma |
e5 |
244 |
0 |
100 |
|
EDTA plasma |
e6 |
401 |
0 |
100 |
|
EDTA plasma |
e7 |
337 |
0 |
100 |
|
EDTA plasma |
e8 |
378 |
0 |
100 |
|
EDTA plasma |
e9 |
424 |
45 |
89 |
|
EDTA plasma |
e10 |
413 |
38 |
91 |
|
EDTA plasma |
e11 |
261 |
5 |
98 |
|
Citrate plasma |
c1 |
269 |
0 |
100 |
|
Citrate plasma |
c2 |
384 |
2 |
99 |
|
Citrate plasma |
c3 |
302 |
4 |
99 |
|
Heparın plasma |
h1 |
307 |
1 |
100 |
|
Heparın plasma |
h2 |
281 |
3 |
99 |
|
Mean |
98 |
|||
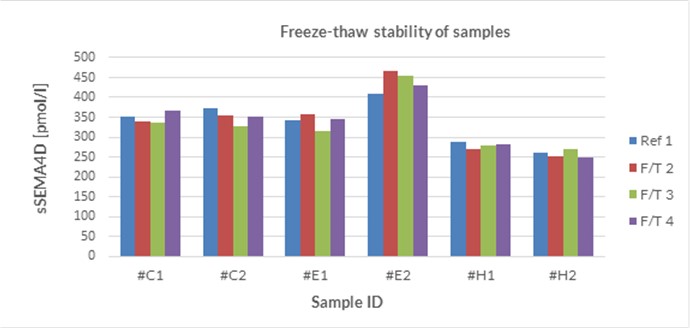
Sample Stability
The stability of endogenous soluble Semaphorin 4D was tested by comparing soluble Semaphorin 4D measurements in samples that had undergone up to four freeze-thaw cycles.
For freeze-thaw experiments, samples were collected according to the supplier’s instruction using blood collection devices and stored at -80°C. Reference samples were freeze-thawed once. The mean recovery of sample concentration after four freeze-thaw cycles (F/T) is 100%.
|
sSEMA4D [pmol/l] |
|
|||||
|
Sample matrix |
ID |
Ref |
2x F/T |
3x F/T |
4x F/T |
% Recovery after 4x F/T |
|
EDTA plasma |
e1 |
351 |
340 |
336 |
366 |
96% |
|
EDTA plasma |
e2 |
372 |
356 |
327 |
351 |
106% |
|
Citrate plasma |
c1 |
343 |
358 |
314 |
345 |
99% |
|
Citrate plasma |
c2 |
410 |
468 |
454 |
429 |
96% |
|
Heparın plasma |
h1 |
287 |
270 |
280 |
282 |
102% |
|
Heparın plasma |
h2 |
261 |
252 |
269 |
250 |
105% |
|
Mean |
100% |
|||||
All samples should undergo a maximum of four freeze-thaw cycles.
Sample Values
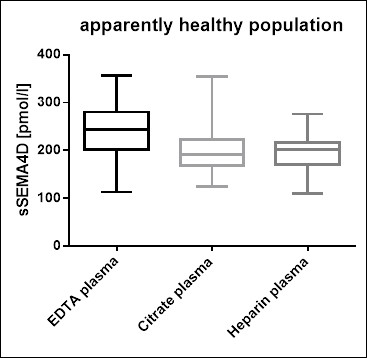
Soluble Semaphorin 4D Values in Apparently Healthy Individuals
To provide expected values for circulating soluble Semaphorin 4D, a panel of samples from apparently healthy donors was tested.
A summary of the results is shown below:
|
sSEMA4D [pmol/l] |
|||||
|
Sample matrix |
n |
Mean |
Median |
Min |
Max |
|
EDTA plasma |
44 |
239 |
245 |
113 |
357 |
|
Heparın plasma |
28 |
194 |
201 |
109 |
276 |
|
Citrate plasma |
43 |
199 |
192 |
125 |
355 |
We recommend establishing the normal range for each laboratory.
The figure below shows a comparison of sample values in different sample matrices.
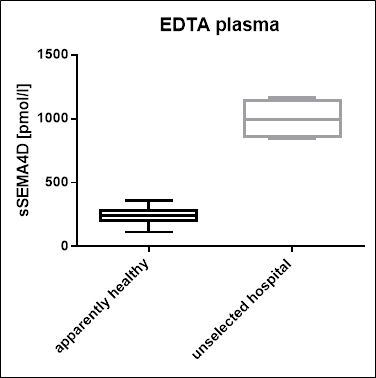
Soluble Semaphorin 4D Values in an Unselected Hospital Panel
In addition to samples from apparently healthy donors, a panel of samples from unselected hospital patients was tested.
A summary of the results is shown below:
|
sSEMA4D [pmol/l] |
|||||
|
Sample matrix |
n |
Mean |
Median |
Min |
Max |
|
EDTA plasma |
4 |
997 |
991 |
841 |
1 165 |
|
Heparın plasma |
13 |
412 |
417 |
326 |
477 |
|
Citrate plasma |
7 |
274 |
268 |
199 |
330 |
A comparison of EDTA plasma samples from an apparently healthy individuals and from an unselected hospital patient cohort shows that soluble Semaphorin 4D is significantly increased in the hospitalized cohort:
|
sSEMA4D [pmol/l] |
|||||
|
Panel |
n |
Mean |
Median |
Min |
Max |
|
Unselected hospital |
4 |
997 |
991 |
841 |
1 165 |
|
Apparenly healthy |
44 |
239 |
245 |
113 |
357 |
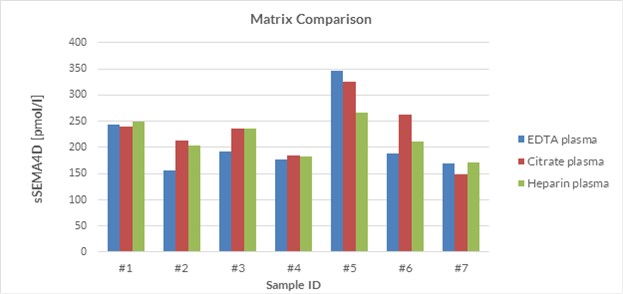
Matrix Comparison
To assess whether all tested matrices behave the same way in the soluble Semaphorin 4D ELISA, concentrations of soluble Semaphorin 4D were measured in EDTA, heparın and citrate plasma samples prepared from apparently healthy donor. Each individual donated blood in all tested sample matrices.
Soluble Semaphorin 4D was measured in plasma samples from seven different individual donors. A summary table of soluble Semaphorin 4D levels in various sample matrices is shown below:
| sSEMA4D [pmol/l] | ||||
| Donor ID | EDTA plasma | Citrate plasma | Heparın plasma | % CV |
| #1 | 244 | 239 | 250 | 2 |
| #2 | 156 | 213 | 204 | 13 |
| #3 | 192 | 237 | 237 | 9 |
| #4 | 177 | 184 | 182 | 2 |
| #5 | 347 | 325 | 266 | 11 |
| #6 | 188 | 262 | 211 | 14 |
| #7 | 169 | 148 | 171 | 6 |
| Mean | 8 | |||
A figure of soluble Semaphorin 4D levels in various sample matrices is shown below:
Why we don’t recommend serum as matrix to measure soluble Semaphorin 4D?
We analyzed soluble SEMA4D in both serum and plasma samples. Based on our results we do not recommend the use of serum as matrix for sSEMA4D analysis. A comparison between sSEMA4D levels in serum and plasma resulted in significantly elevated sSEMA4D levels in serum. This can be explained that plasma anticoagulants prohibit coagulation-induced platelet activation that might lead to sSEMA4D shedding. Zhu L and colleagues (2013. Blood 16, 4221–4230) demonstrated that blood coagulation-related platelet activation, e.g. due to vascular injury during sample collection, leads to increased sSEMA4D surface expression, followed by shedding into the circulation. We could demonstrate that plasma is free of shed sSEMA4D and is a suitable matrix for reproducible quantification of soluble Semaphorin 4D (Laber A et al., 2019. Anal. Biochem. 574, 15–22.).
-
Hypoglycaemia leads to a delayed increase in platelet and coagulation activation markers in people with type 2 diabetes treated with metformin only: Results from a stepwise hypoglycaemic clamp study. Aberer, F., Pferschy, P.N., Tripolt, N.J., Sourij, C., Obermayer, A.M., Prüller, F., Novak, E., Reitbauer, P., Kojzar, H., Prietl, B., Kofler, S., Brunner, M., Svehlikova, E., Stojakovic, T., Scharnagl, H., Oulhaj, A., Aziz, F., Riedl, R., Sourij, H., 2020. Diabetes, Obesity and Metabolism 22, 212–221.
- Sandwich ELISA for the Quantification of Soluble Human Semaphorin 4D, a factor promoting skeletal metastases. Bitzer, A., Laber, A., Gadermaier, E., Wallwitz, J., Berg, G., Himmler, G., 2019, in: Osteologie. Presented at the OSTEOLOGIE 2019, Georg Thieme Verlag KG, p. V 1.8.
- Plasma levels of Semaphorin 4D are decreased by adjuvant tamoxifen but not aromatase inhibitor therapy in breast cancer patients.
Göbel, A., Kuhlmann, J.D., Link, T., Wimberger, P., Link-Rachner, C., Thiele, S., Dell’Endice, S., Furesi, G., Breining, D., Rauner, M., Hofbauer, L.C., Rachner, T.D., 2019. Journal of Bone Oncology, 16: 100237. PMID: 31011525
- A high-sensitivity enzyme immunoassay for the quantification of soluble human semaphorin 4D in plasma.
Laber, A., Gadermaier, E., Wallwitz, J., Berg, G., Himmler, G., 2019. Anal. Biochem. 574, 15–22.
PMID: 30879960
 Download biomedica product list
Download biomedica product list