Mouse/Rat Angiopoietin-2 ELISA Kit | BI-ANG2MR
-
Method
Sandwich ELISA, HRP/TMB, 12×8-well detachable strips
-
Sample type
Mouse or rat serum, plasma
-
Sample volume
5 µl / well
-
Assay time
2 h / 2 h / 1 h / 30 min
-
Sensitivity
18.3 pmol/l (= 1 005 pg/ml)
-
Standard range
0 – 1400 pmol/l (0 – 76,860 pg/ml)
-
Conversion factor
1 pmol/l = 54.9 pg/ml (MW: 54.9 kDa)
-
Precision
In-between-run (n=4) ≤ 9 %
Within-run (n=5) ≤ 4 %
-
Specificity
Endogenous and recombinant mouse/rat Angiopoietin-2.
-
Cross-reactivity
No cross-reactivity with Angiopoietin-1.
-
Use
Research use only.
-
Validation Data
See validation data tab for: precision, accuracy, diltuion linearity etc.
Mouse/Rat Ang2 ELISA Product Overview
The mouse/rat Angiopoietin-2 ELISA kit is a 5.5 h, 96-well sandwich ELISA for the quantitative determination of Angiopoietin-2 in mouse and rat serum and plasma.
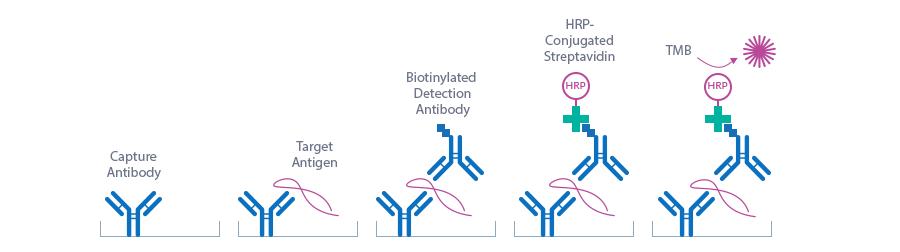
Mouse/Rat Ang2 ELISA Assay Principle
The figure below explains the principle of the Mouse Angiopoietin-2 ELISA and Rat Angiopoietin-2 ELISA:
Capture antibody: monoclonal Angiopoietin-2 antibody
Detection antibody: polyclonal Angiopoietin-2 antibody
Target antigen: mouse Angiopoietin-2
In a first step, pre-diluted standard/control/sample are pipetted into the wells of the microtiter strips, which are pre-coated with a recombinant monoclonal Angiopoietin-2 antibody. Angiopoietin-2 present in the sample binds to the pre-coated antibody in the well. After a first wash step, which removes non-specifically unbound material, detection antibody is added and forms a sandwich with the antigen bound on the plate. After another washing step the conjugate (streptavidin-HRP) is pipetted into the wells and reacts with the detection antibody. After another washing step, the substrate (TMB, tetramethylbenzidine) is pipetted into the wells. The enzyme catalysed color change of the substrate is directly proportional to the amount of mouse/rat Angiopoietin-2 present in the sample. This color change is detectable with a standard microtiter plate ELISA reader. The concentration of Angiopoietin-2 in the sample is determined directly from the dose response curve.
The Ang-2 ELISA kit utilizes recombinant mouse Angiopoetin-2 as a calibrator. Mouse, rat, bovine and human Angiopoietin-2 share a high homology (>85%).
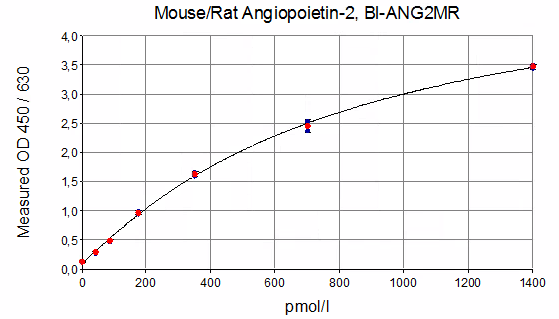
Mouse/Rat Ang2 ELISA Typical Standard Curve
The figure below shows a typical standard curve for the Mouse Angiopoietin 2 ELISA and Rat Angiopoietin 2 ELISA. The immunoassay is calibrated against recombinant Angiopoietin-2 protein:
Mouse/Rat Ang2 ELISA Kit Components
|
Contents |
Description |
Quantity |
|
PLATE |
Detachable microtiter strips pre-coated with recombinant monoclonal Angiopoietin-2 antibody |
12 x 8 tests |
|
WASHBUF |
Wash buffer concentrate 20x, natural cap |
1 x 50 ml |
|
STOCK STD |
Stock standard containing 1400 pmol/l of recombinant mouse Angiopoietin-2, red cap, lyophilised |
1 vial |
|
CTRL |
Control A and B, yellow cap, lyophilized, exact concentrations see labels |
1 vial |
|
ASYBUF |
Assay buffer, red cap, ready to use |
1 x 15 ml |
|
AB |
Polyclonal Angiopoietin-2 antibody, biotinylated, green cap, ready to use |
1 x 13 ml |
|
CONJ |
Streptavidin-HRPO conjugate, amber cap, ready to use |
1 x 13 ml |
|
SUB |
Substrate (TMB solution), blue cap, ready to use |
1 x 13 ml |
|
STOP |
Stop solution, white cap, ready to use |
1 x 7 ml |
Storage instructions: all reagents of the Angiopoietin-2 Mouse/Rat ELISA kit are stable at 4°C (2-8°C) until the expiry date stated on the label of each reagent.
Serum and plasma are suitable for use in this Ang2 ELISA kit. We recommend duplicate measurements for all samples, standards and controls. Do not change sample type during studies. The sample collection and storage conditions listed are intended as general guidelines.
Serum & Plasma
Collect venous blood samples by using standardized blood collection tubes for serum or plasma. Perform serum and plasma separation by centrifugation according to supplier’s instructions of the blood collection devices and measure the acquired serum or plasma samples as soon as possible. For longer storage aliquot and store samples at -25°C or lower. Samples are stable for at least four freeze-thaw cycles. Thawed samples should be assayed as soon as possible. Lipemic or haemolyzed samples may give erroneous results.
Samples with values above STD7 (1400 pmol/l) can be diluted with ASYBUF (Assay buffer).
Serum and plasma are suitable for use in this assay. Do not change sample type during studies.
Reagent Preparation
Wash Buffer
|
1. |
Bring the WASHBUF concentrate to room temperature. Crystals in the buffer concentrate will dissolve at room temperature. |
|
2. |
Dilute the WASHBUF concentrate 1:20, e.g. 50 ml WASHBUF + 950 ml distilled or deionized water. Only use diluted WASHBUF when performing the assay. |
The diluted WASHBUF is stable up to one month at 4°C (2-8°C).
Control
|
1. |
Pipette 100 µl of distilled or deionized water into the control (CTRL) vial. The exact concentration is stated on the label of the vial. |
|
2. |
Leave at room temperature (18-26°C) for 15 min. Vortex gently. |
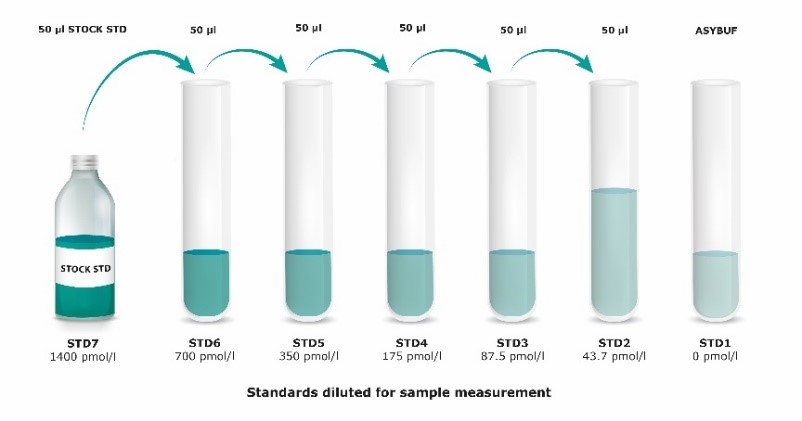
Standards
|
1. |
Reconstitute the STOCK STD (stock standard) in 100 µl deionized water. Leave at room temperature (18-26°C) for 15 min and mix well prior to making dilutions. |
|
2. |
Mark tubes STD6 to STD1. Use polypropylene tubes. Mark STOCK STD as STD7. |
|
4. |
Pipette 50 µl of assay buffer into tubes marked as STD6 to STD1. |
|
6. |
Prepare a two-fold serial dilution to obtain STD6 to STD2. Pipette 50 µl of the reconstituted STOCK STD into the tube labeled STD6. Mix thoroughly. Continue serial dilutions for STD5, STD4, STD3, STD2. |
|
7. |
ASYBUF serves as STD1 (= 0 nmol/l mouse Angiopoeitin-2). |
Reconstituted STOCK STD is stable at -25°C or lower until expiry date stated on the label.
Sample Preparation
Bring samples to room temperature and mix samples gently to ensure the samples are homogeneous. We recommend duplicate measurements for all samples.
Mouse/Rat Ang2 ELISA Assay Protocol
Read the entire protocol before beginning the assay.
|
1. |
Bring samples and reagents to room temperature (18-26°C). |
|
2. |
Mark positions for STD/CTRL/SAMPLE (standard/control/sample) on the protocol sheet. |
|
3. |
Take microtiter strips out of the aluminum bag. Store unused strips with desiccant at 4°C in the aluminum bag. Strips are stable until expiry date stated on the label. |
|
4. |
Pipette 100 µl ASYBUF (assay buffer, red cap) into each well. |
|
5. |
Add 5 µl STD/CTRL/SAMPLE in duplicates into the respective wells, swirl gently. |
|
6. |
Cover the plate tightly and incubate for 2 hours at room temperature (18-26°C) in the dark. |
|
7. |
Aspirate and wash wells 5x with 300 μl diluted wash buffer. After final wash, remove remaining wash buffer by strongly tapping plate against paper towel. |
|
8. |
Add 100 µl AB (biotinylated anti-Angiopoietin-2 antibody, green cap) into each well, swirl gently. |
|
9. |
Cover the plate tightly and incubate for 2 hours at room temperature (18-26°C) in the dark. |
|
10. |
Aspirate and wash wells 5x with 300 µl diluted WASHBUF (wash buffer). After final wash, remove remaining WASHBUF by strongly tapping plate against paper towel. |
|
11. |
Add 100 µl CONJ (conjugate, amber cap) into each well, swirl gently. |
|
12. |
Cover the plate tightly and incubate for 1 hour at room temperature (18-26°C) in the dark. |
|
13. |
Aspirate and wash wells 5x with 300 µl diluted WASHBUF (wash buffer). After final wash, remove remaining WASHBUF by strongly tapping plate against a paper towel. |
|
14. |
Add 100 µl SUB (substrate, blue cap) into each well, swirly gently. |
|
15. |
Incubate for 30 min at room temperature (18-26°C) in the dark. |
Calculation of Results
Read the optical density (OD) of all wells on a plate reader using 450 nm wavelength (reference wavelength 630 nm). Construct a standard curve from the absorbance read-outs of the standards using commercially available software capable of generating a four-parameter logistic (4-PL) fit. Alternatively, plot the standards’ concentration on the x-axis against the mean absorbance for each standard on the y-axis and draw a best fit curve through the points on the graph. Curve fitting algorithms other than 4-PL have not been validated and need to be evaluated by the user. Obtain sample concentrations from the standard curve. If required, pmol/l can be converted into pg/ml by applying a conversion factor (1 pg/ml = 0.018 pmol/l; Mouse Angiopoietin-2 MW: 54.9 kDa). Respective dilution factors have to be considered when calculating the final concentration of the sample.
The quality control (QC) protocol supplied with the kit shows the results of the final release QC for each kit lot. Data for OD obtained by customers may differ due to various influences and/or due to the normal decrease of signal intensity during shelf life. However, this does not affect validity of results as long as an OD of 1.00 or more is obtained for the STD with the highest concentration and the value of the CTRL is in range (target range see label).
Angiopoietin-2 Protein
Angiopoietin-2 (ANG2) is a 56.9 kDa glycosylated growth factor that is specific for endothelial cells (ECs). ANG2 is expressed in embryonic vessels and contributes to the formation of new vasculature. In adults, it is restricted to sites of vascular remodeling (e.g. ovary, uterus, placenta) and wound healing. ANG2 is regulated by the cytokine vascular endothelial growth factor (VEGF). Together with VEGF, ANG2 induces endothelial cell migration, proliferation, and vascular sprouting. During angiogenesis, ANG2 exerts its effects via the angiopoietin-1/TIE2 receptor signaling system on endothelial cells. Disruption of this signaling leads to the loss of endothelial integrity. In consequence, the endothelium responds to various pro-inflammatory cytokines and growth factors. Thus, ANG2 might cause vascular micro-inflammation in patients with chronic kidney disease (CKD). Various studies demonstrated that ANG2 levels increase with CKD stage and are associated with fluid overload and abnormal cardiac structure. Furthermore, ANG2 concentrations correlate with mortality in patients with CKD stages 4–5. Although ANG2 levels recover after successful kidney transplantation, ANG2 continues to be a cardiovascular risk factor in this population.
In cancer, targeting the TIE2-Angiopoietin pathway has shown promising results in some pre-clinical and clinical trials, including studies on recurrent or metastatic breast and renal cell carcinomas.
|
Molecular weight |
56.9 kDa |
|
Cellular localization |
Extracellular, plasma membrane |
|
Post-translational modifications |
Glycosylation, lipidation (GPI-anchor) |
|
Alternative names |
ANG2, ANGPT2, Angiopoietin 2, Angiopoietin-2, ANG-2, Angiopoietin-2B, Angiopoietin-2a, Tie2-Ligand |
|
Entrez/NCBI ID |
|
|
Genecards |
|
|
OMIM |
|
|
Protein Atlas |
|
|
Uniprot ID |
Areas of interest:
- Ischemic pathologies (PAD, CAD)
- Inflammation (Bowel disease, Chron’s disease, cirrhosis, sepsis)
- Autoimmune disease (rheumatoid arthritis, psoriasis)
- Artherosclerosis
- Chronic kidney disease
- Diabetic retinopathy
- Cancer
- Biomedical significance of endothelial cell specific growth factor, angiopoietin.
Koh, G.Y., Kim, I., Kwak, H.J., Yun, M.J., Leem J.C., 2002. Exp. Mol. Med., 34(1):1–11.
PMID:11989972
- Control of vascular morphogenesis and homeostasis through the angiopoietin–Tie system.
Augustin, H.G., Koh, G.Y., Thurston G., Alitalo, K., 2009. Nat. Rev. Mol. Cell. Biol., 10(3): 165–177.
PMID:19234476
- Crystal structures of the Tie2 receptor ectodomain and the angiopoietin-2–Tie2 complex.
Barton, W.A., Tzvetkova-Robev, D., Miranda, E.P., Kolev, M.V., Rajashankar, K.R., Himanen, J.P., Nikolov, D.B., 2006; Nat. Struct. Mol. Biol., 13(6): 524–532.
PMID:16732286
- Characterization and Expression of a Novel Alternatively Spliced Human Angiopoietin-2.
Kim, I., Kim, J.H., Ryu, Y.S., Jung, S.H., Nah, J.J., Koh, G.Y., 2000. J. Biol. Chem., 275(24): 18550–18556.
PMID:10766762
- Angiopoietin-1 and Angiopoietin-2 Inhibitors: Clinical Development.
Gillen, J., Richardson, D., Moore, K., 2019. Curr. Oncol. Rep., 21(3): 22.
PMID:30806847
- Targeting the Angiopoietin-2/Tie-2 axis in conjunction with VEGF signal interference.
Biel, N.M., Siemann D.W., 2016. Cancer Lett., 380(2): 525–533.
PMID:25312939
- Angiopoietin-2: a multifaceted cytokine that functions in both angiogenesis and inflammation: Angiopoietin-2 and inflammation.
Scholz, A., Plate, K.H., Reiss, Y. 2015. Ann. N. Y. Acad. Sci., 1347(1): 45–51.
PMID:25773744
- Circulating Angiopoietin-2 levels predict mortality in kidney transplant recipients: a 4-year prospective case-cohort study.
Molnar, M.Z., Kümpers, P., Kielstein, J.T., Schiffer, M., Czira, M.E., Ujszaszi, A., Kovesdy, C.P., Mucsi, I., 2014; Transpl. Int., 27(6): 541–552.
PMID:24628855
- Circulating angiopoietin-2 levels increase with progress of chronic kidney disease.
David, S., Kümpers, P., Lukasz, A., Fliser, D., Martens-Lobenhoffer, J., Bode-Böger, S.M., Kliem, V., Haller, H., Kielstein, J.T., 2010. Nephrol Dial Transplant, 25: 2571-2579.
PMID:20179005
- The interaction between fluid status and angiopoietin-2 in adverse renal outcomes of chronic kidney disease.
Tsai, Y.C., Chiu, Y.W., Kuo, H.T., Lee J.J., Lee, S.C., Chen, T.H., Lin, M.Y., Hwang, S.J., Kuo, M.C., Hsu, Y.L., Chen, H.C., 2017. PLoS One, 12 (3): e173906.
PMID: 28333979
- Angiopoietin-2, Angiopoietin-1 and subclinical cardiovascular disease in Chronic Kidney Disease.
Tsai, Y.C., Lee, C.S., Chiu, Y.W., Kuo, H.T., Lee, S.C., Hwang, S.J., Kuo, M.C., Chen, H.C., 2016. Scientific Reports, 6: 39400.
PMID:27991547
- Angiopoietin-1 and Angiopoietin-2 Inhibitors: Clinical Developments.
Gillen, J., Richardson, D., Moore, K., 2019. Current Oncology Reports, 21: 22.
PMID:30806847
All Biomedica ELISAs are validated according to international FDA/ICH/EMEA guidelines. For more information about our validation guidelines, please refer to our quality page and published validation guidelines and literature.
- ICH Q2(R1) Validation of Analytical Procedures: Text and Methodology.
- EMEA/CHMP/EWP/192217/2009 Guideline on bioanalytical method validation.
- Bioanalytical Method Validation, Guidance for Industry, FDA, May 2018
Calibration
The Angiopoietin-2 Mouse/Rat ELISA is calibrated against mouse Angiopoietin-2 protein (Uniprot O35608).
Mouse/Rat Ang2 ELISA Detection Limit & Sensitivity
To determine the sensitivity of the Angiopoietin-2 Mouse/Rat ELISA, experiments measuring the lower limit of detection (LOD) and the lower limit of quantification (LLOQ) were conducted.
The LOD, also called the detection limit, is the lowest point at which a signal can be distinguished above the background signal, i.e. the signal that is measured in the absence of mouse Angiopoietin-2, with a confidence level of 99%. It is defined as the mean back calculated concentration of standard 1 (0 pmol/l of mouse Angiopoietin-2, five independent measurements) plus three times the standard deviation of the measurements.
The LLOQ, or sensitivity of an assay, is the lowest concentration at which an analyte can be accurately quantified. The criteria for accurate quantification at the LLOQ are an analyte recovery between 75 and 125% and a coefficient of variation (CV) of less than 25%. To determine the LLOQ, standard 2, i.e. the lowest standard containing mouse Angiopoietin-2, is diluted, measured five times and its concentration is back calculated. The lowest dilution, which meets both criteria, is reported as the LLOQ.
The following values were determined for the Angiopoietin-2 Mouse/Rat ELISA:
|
LOD |
18.3 pmol/l |
|
LLOQ |
21.9 pmol/l |
Mouse/Rat Ang2 ELISA Precision
The precision of an ELISA is defined as its ability to measure the same concentration consistently within the same experiments carried out by one operator (within-run precision or repeatability) and across several experiments using the same samples but conducted by several operators using different ELISA lots (in-between-run precision or reproducibility).
Within-Run Precision
Within-run (intra-assay) precision was assessed by measuring two samples of known concentrations five times within one Angiopoietin-2 Mouse/Rat ELISA kit lot by one operator.
|
ID |
n |
Mean Angiopoietin-2 [pmol/l] |
SD [pmol/l] |
CV (%) |
|
Sample 1 |
5 |
77 |
3.0 |
4 |
|
Sample 2 |
5 |
120 |
2.2 |
2 |
In-Between-Run Precision
In-between-run (inter-assay) precision was assessed by measuring two samples of known concentrations four times within three Angiopoietin-2 Mouse/Rat ELISA lots by two operators.
|
ID |
n |
Mean Angiopoietin-2 [pg/ml] |
SD [pg/ml] |
CV (%) |
|
Sample 1 |
4 |
144 |
4.5 |
3 |
|
Sample 2 |
4 |
182 |
16.6 |
9 |
Mouse/Rat Ang2 ELISA Accuracy
The accuracy of an ELISA is defined as the precision with which it can recover samples of known concentrations.
The recovery of the Angiopoietin-2 human ELISA was measured by adding recombinant Angiopoietin-2 to mouse and rat samples containing a known concentration endogenous Angiopoietin-2. The % recovery of the spiked concentration was calculated as the percentage of measured compared over the expected value.
The tables shows the summary of the recovery experiments in the Angiopoietin-2 Mouse /Rat ELISA in human samples:
|
% Recovery |
|||||
|
+150 pmol/l |
+750 pmol/l |
||||
|
Sample matrix |
n |
Mean |
Range |
Mean |
Range |
|
Mouse |
4 |
101 |
93-107 |
94 |
89-99 |
|
Rat |
3 |
100 |
74-121 |
94 |
91-98 |
Data showing % recovery of recombinant Angiopoietin-2 in mouse serum samples:
|
Angiopoietin-2 [pmol/l] |
% Recovery |
|||||
|
Sample matrix |
ID |
Reference |
+ 150 pmol/l |
+ 750 pmol/l |
+ 150 pmol/l |
+ 750 pmol/l |
|
Mouse Serum |
ms1 |
180 |
326 |
807 |
107 |
96 |
|
Mouse Serum |
ms2 |
176 |
312 |
832 |
101 |
99 |
|
Mouse Serum |
ms3 |
133 |
276 |
752 |
103 |
91 |
|
Mouse Serum |
ms4 |
147 |
275 |
744 |
93 |
89 |
|
Mean |
101 |
94 |
||||
|
Min |
93 |
89 |
||||
|
Max |
107 |
99 |
||||
Data showing % recovery of recombinant Angiopoietin-2 in human rat plasma samples:
|
Angiopoietin-2 [pmol/l] |
% Recovery |
|||||
|
Sample matrix |
ID |
Reference |
+ 40 pmol/l |
+ 200 pmol/l |
+ 40 pmol/l |
+ 200 pmol/l |
|
Rat plasma |
rp1 |
185 |
350 |
827 |
121 |
98 |
|
Rat plasma |
rp2 |
141 |
289 |
770 |
106 |
93 |
|
Rat plasma |
rp3 |
138 |
238 |
749 |
74 |
91 |
|
Mean |
100 |
94 |
||||
|
Min |
74 |
91 |
||||
|
Max |
121 |
98 |
||||
Mouse/Rat Ang2 ELISA Dilution Linearity & Parallelism
Tests of dilution linearity and parallelism ensure that both recombinant and endogenous samples containing Angiopoietin-2 (ANG2) behave in a dose dependent manner and are not affected by matrix effects. Dilution linearity assesses the accuracy of measurements in diluted human samples spiked with known concentrations of recombinant analyte. By contrast, parallelism refers to dilution linearity in human samples and provides evidence that the endogenous analyte behaves in the same way as the recombinant one. Dilution linearity and parallelism are assessed for each sample type and should be within 20% of the expected concentration.
Dilution Linearity
Dilution linearity was assessed by serially diluting samples spiked with 650 pmol/l or 750 pmol/l recombinant mouse Angiopoietin-2 with assay buffer.
The table below shows the mean recovery and range of serially diluted recombinant Angiopoietin-2 :
|
|
% Recovery of recombinant Angiopoietin-2 in diluted samples |
||||||
|
|
1+1 |
1+3 |
1+7 |
||||
|
Sample matrix |
n |
Mean |
Range |
Mean |
Range |
Mean |
Range |
|
Mouse |
4 |
103 |
87-116 |
116 |
112-119 |
113 |
98-122 |
|
Rat |
3 |
90 |
88-92 |
79 |
10-84 |
- |
- |
Data showing dilution linearity of 650 pmol/l recombinant mouse Angiopoietin-2 (ANG2) spiked into mouse serum samples (ref) containing endogenous ANG2:
|
Angiopoietin-2 [pmol/l] |
Recovery (%) |
|||||||
|
Sample matrix |
ID |
Reference |
1+1 |
1+3 |
1+7 |
1+1 |
1+3 |
1+7 |
|
Mouse serum |
ms1 |
594 |
278 |
166 |
73 |
94 |
112 |
98 |
|
Mouse serum |
ms2 |
596 |
259 |
172 |
91 |
87 |
116 |
122 |
|
Mouse serum |
ms3 |
533 |
308 |
155 |
80 |
116 |
116 |
120 |
|
Mouse serum |
ms4 |
571 |
331 |
169 |
82 |
116 |
119 |
115 |
|
|
|
|
|
Mean |
103 |
116 |
113 |
|
|
|
|
|
|
Min |
87 |
112 |
98 |
|
|
|
|
|
|
Max |
116 |
119 |
122 |
|
Data showing dilution linearity of 750 pmol/l recombinant mouse Angiopoietin-2 (ANG2) spiked into rat plasma (ref) containing endogenous ANG2:
|
Angiopoietin-2 [pmol/l] |
Recovery (%) |
|||||
|
Sample matrix |
ID |
Reference |
1+1 |
1+3 |
1+1 |
1+3 |
|
Rat plasma |
rp1 |
827 |
381 |
174 |
92 |
84 |
|
Rat plasma |
rp2 |
770 |
338 |
135 |
88 |
70 |
|
Rat plasma |
rp3 |
749 |
335 |
157 |
89 |
84 |
|
|
|
|
|
Mean |
90 |
79 |
|
|
|
|
|
Min |
88 |
70 |
|
|
|
|
|
Max |
92 |
84 |
Parallelism
Parallelism was assessed by serially diluting serum samples containing endogenous Angiopoietin 2 with assay buffer.
The table below shows the mean recovery and range of serially diluted endogenous Angiopoietin-2 in mouse and rat samples:
|
|
% Recovery of recombinant Angiopoietin-2 in diluted samples |
||||||
|
|
1+1 |
1+3 |
1+7 |
||||
|
Sample matrix |
n |
Mean |
Range |
Mean |
Range |
Mean |
Range |
|
Mouse |
4 |
100 |
96-104 |
102 |
97-110 |
101 |
87-109 |
|
Rat |
3 |
92 |
83-98 |
94 |
89-99 |
104 |
98-112 |
Data showing dilution linearity of mouse serum samples containing endogenous Angiopoietin-2:
|
Angiopoietin-2 [pmol/l] |
Recovery (%) |
|||||||
|
Sample matrix |
ID |
Reference |
1+1 |
1+3 |
1+7 |
1+1 |
1+3 |
1+7 |
|
Mouse serum |
ms1 |
449 |
233 |
123 |
61 |
104 |
110 |
109 |
|
Mouse serum |
ms2 |
207 |
104 |
52 |
22 |
100 |
100 |
87 |
|
Mouse serum |
ms3 |
143 |
69 |
35 |
19 |
96 |
97 |
108 |
|
|
|
|
|
Mean |
100 |
102 |
101 |
|
|
|
|
|
|
Min |
96 |
97 |
87 |
|
|
|
|
|
|
Max |
104 |
110 |
109 |
|
Data showing dilution linearity of rat plasma samples containing endogenous Angiopoietin-2:
|
Angiopoietin-2 [pmol/l] |
Recovery (%) |
|||||||
|
Sample matrix |
ID |
Reference |
1+1 |
1+3 |
1+7 |
1+1 |
1+3 |
1+7 |
|
Rat plasma |
rp1 |
147 |
72 |
36 |
19 |
98 |
99 |
102 |
|
Rat plasma |
rp2 |
150 |
68 |
33 |
18 |
90 |
89 |
98 |
|
Rat plasma |
rp3 |
108 |
53 |
27 |
14 |
98 |
99 |
104 |
|
Rat plasma |
rp4 |
145 |
60 |
33 |
20 |
83 |
91 |
112 |
|
|
|
|
|
Mean |
92 |
94 |
104 |
|
|
|
|
|
|
Min |
83 |
89 |
98 |
|
|
|
|
|
|
Max |
98 |
99 |
112 |
|
Mouse/Rat Ang2 ELISA Specificity
The specificity of an ELISA is defined as its ability to exclusively recognize the analyte of interest.
Competition of Signal
Competition experiments were carried out by pre-incubating mouse and rat samples with an excess of coating antibody. The concentration measured in this mixture was then compared to a reference value, which was obtained from the same sample but without the pre-incubation step. Mean competition was 98%.
|
Angiopoietin-2 [pmol/l] |
|
||
|
ID |
Reference |
Reference + capture AB |
% Competition |
|
m1 |
449 |
6 |
99 |
|
m2 |
207 |
0 |
100 |
|
m3 |
61 |
0 |
100 |
|
m4 |
143 |
0 |
100 |
|
r1 |
188 |
19 |
90 |
|
r2 |
145 |
0 |
100 |
|
r3 |
142 |
0 |
100 |
|
r4 |
146 |
6 |
96 |
Sample and Standard Stability
Serum and plasma are suitable for use in this assay. Do not change sample type during studies. We recommend duplicate measurements for all samples, standards and controls. The sample collection and storage conditions listed are intended as general guidelines.
Freeze-thaw Stability
The stability of endogenous Angiopoietin-2 was tested by comparing measurements in blood samples that had undergone 4 freeze-thaw cycles (F/T). The mean recovery of sample concentration after 4 freeze-thaw cycles is 96%. Samples can undergo at least up to 4 freeze-thaw cycles.
Standard Stability
The stability of recombinant mouse Angiopoietin-2 (ANG2) was tested by comparing 3 measurements of standards spiked to different values that had undergone 5 freeze-thaw cycles. The mean recovery of standard concentration after 5 freeze-thaw cycles is 99%. Standards can undergo at least up to 5 freeze-thaw cycles.
The stability of recombinant mouse Angiopoietin-2 (ANG2) was tested by comparing 3 measurements in standards spiked to different values that was tested for bench top stability for 2h, 4h and 20h at room temperature (18-26°C). The mean recovery of standard concentration after 20 hours at room temperature is 99%.
Sample Values
Angiopoietin-2 Values
To provide reference values for circulating mouse and rat Angiopoietin-2, a panel of samples was tested.
A summary of the results is shown below:
|
|
|
Angiopoietin-2 [pmol/l] | ||
|
Sample matrix |
n |
Mean |
Range |
Median |
|
Mouse serum |
18 |
132 |
57-457 |
105 |
|
Mouse plasma |
7 |
133 |
94-207 |
127 |
|
Rat serum |
8 |
293 |
220-354 |
292 |
|
Rat plasma |
11 |
136 |
107-178 |
127 |
 Download biomedica product list
Download biomedica product list