BNP Fragment EIA | BI-20852W
-
Method
Competitive enzyme immunoassay (EIA), HRP/TMB, 12×8-well detachable strips
-
Sample type
Serum, EDTA plasma, heparın plasma, citrate plasma
-
Product volume
30 µl / well
-
Assay time
Overnight (18 – 25 h) / 20 min
-
Sensitivity
171 pmol/l (=1449 pg/ml)
-
Standard range
0 – 6,400 pmol/l (0 – 54,237 pg/ml)
-
Conversion factor
1 pmol/l = 8.475 pg/ml (MW: 8.475 kDa)
-
Specificity
Endogenous and recombinant human NT-proBNP.
-
Precision
In-between-run (n=8): ≤ 7 % CV
Within-run (n=3): ≤ 3 % CV
-
Cross-reactivity
The assay does not cross react with rat, mouse, dog, or cat samples.
-
Use
Research use only
-
Validation Data
See validation data tab for: precision, accuracy, dilution linearity, values for healthy donors, etc
NT-proBNP Test Kit Product Overview
The NT-proBNP test kit is an overnight, 96-well competitive enzyme immunoassay (EIA). This NT-proBNP assay utilizes one antibody that binds to the amino acid region of 8-29 of the NT-proBNP peptide. Thus, the BNP fragment immunoassay is for the quantitative determination of NT-proBNP (1-76), including all NT-proBNP derived fragments containing the amino acid sequence between AA 8-29, in human serum and plasma.
NT-proBNP Test Kit Assay Principle
This NT pro BNP test kit is a competitive enzyme immunoassay (EIA) for the quantitative determination of NT-proBNP (1-76) and NT-proBNP fragments containing AA 8-29 in human serum, EDTA plasma, citrate plasma and heparın plasma.
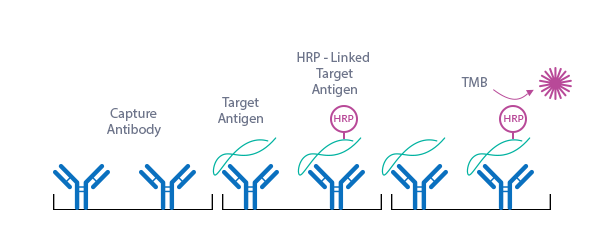
The figure below explains the principle of the BNP fragment EIA:
In a first step, standard/control/sample and conjugate (synthetic NT-proBNP-HRP) are pipetted into the wells of the microtiter strips, which are pre-coated with anti-BNP fragment (AA8-29) antibody. BNP-fragment present in the standard/control/sample and the BNP fragment conjugate binds to the pre-coated antibody in the well. Since BNP fragments in the standard/control/sample and conjugate compete for the antibodies’ binding sites, the relative fraction of unlabeled BNP fragment is proportional to the concentration of BNP fragment in the standard/control/sample. In a washing step all non-specific unbound material is removed. In a second step, the substrate (TMB, tetramethylbenzidine) is pipetted into the wells. The enzyme-catalyzed color change of the substrate is inversely proportional to the amount of BNP fragment in the standard/control/sample. This color change is detectable with a standard microtiter plate reader. A dose response curve of the absorbance (optical density, OD at 450 nm) vs. standard concentration is generated, using the values obtained from the standard. The concentration of BNP fragment in the sample is determined directly from the dose response curve.
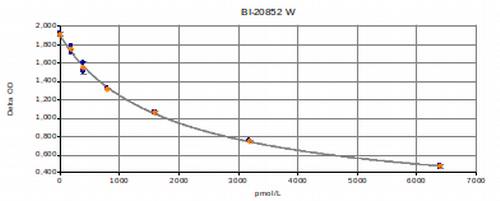
NT-proBNP Test Kit Typical Standard Curve
The figure below shows a typical standard curve for the BNP assay. The immunoassay is calibrated against recombinant NT-proBNP peptide:
NT-proBNP Test Kit Components
|
CONT |
KIT COMPONENTS |
QUANTITY |
|
PLATE |
Polyclonal anti-BNP fragment (AA 8-29) antibody pre-coated microtiter strips in strip holder, packed in aluminum bag with desiccant |
12 x 8 tests |
|
WASHBUF |
Wash buffer concentrate 20x, natural cap |
1 x 50 ml |
|
STD |
Standards 1-7, (0; 200; 400; 800; 1,600; 3,200; 6,400 pmol/l), synthetic human NT-proBNP in human serum, white caps, lyophilized |
7 vials |
|
CTRL |
Control, yellow cap, synthetic human NT-proBNP, lyophilized, exact concentration after reconstitution see label |
1 vial |
|
ASYBUF |
Assay buffer, red cap, ready to use |
1 x 20 ml |
|
CONJ |
Conjugate, (synthetic NT-proBNP-HRP), red dye, amber cap, ready to use |
1 x 6 ml |
|
SUB |
Substrate (TMB solution), blue cap, ready to use |
1 x 22 ml |
|
STOP |
Stop solution, white cap, ready to use |
1 x 7 ml |
Storage instructions: All reagents of the human BNP fragment EIA kit are stable at +4°C (+2-8 °C) until the expiry date stated on the label of each reagent.
Sample Collection & Storage
Serum, EDTA plasma,citrate plasma and heparın plasma are suitable for use in this NT proBNP test. Do not change sample type during studies. We recommend duplicate measurements for all samples, standards and controls. The sample collection and storage conditions listed are intended as general guidelines.
Serum & Plasma
Collect venous blood samples in standardized serum separator tubes (SST) or standardized blood collection tubes using EDTA, heparın or citrate as an anticoagulant. For serum samples, allow samples to clot for 30 minutes at room temperature. BNP fragments are stable in whole blood, serum or plasma for several hours at room temperature or +4°C (+2-8°C). Nevertheless, perform serum and plasma separation by centrifugation according to tube manufacturer’s instructions for use. Assay the acquired samples immediately or aliquot and store at -25°C or lower. Lipemic or haemolyzed samples may give erroneous results. Samples can be subjected to five freeze-thaw cycles.
Reagent Preparation
Wash Buffer
|
1. |
Bring the WASHBUF concentrate to room temperature. Crystals in the buffer concentrate will dissolve at room temperature. |
|
2. |
Dilute the WASHBUF concentrate 1:20, e.g. 50 ml WASHBUF + 950 ml distilled or deionized water. Only use diluted WASHBUF when performing the assay. |
The diluted WASHBUF is stable up to one month at +4°C (+2-8°C).
Standards & Control for Serum and Plasma Measurements
|
1. |
Pipette 200 µl of distilled or deionized water into each standard (STD) and control (CTRL) vial. The standard and control concentrations are printed on the label of each vial. |
|
2. |
Leave at room temperature (+18-26°C) for 20 min. Swirl gently. |
Reconstituted STDs and CTRL are stable at -25°C or lower until expiry date stated on the label. Avoid freeze-thaw cycles.
Sample Preparation
Bring samples to room temperature and mix samples gently to ensure the samples are homogenous. We recommend duplicate measurements for all samples.
Samples for which the OD value is below highest point of the standard range can be diluted with STD 1 (0 pmol/l) or BNP fragment negative human serum.
NT-proBNP Test Kit Assay Protocol
Read the entire protocol before beginning the assay.
|
1. |
Bring samples and reagents to room temperature (+18-26°C). |
|
2. |
Mark position for STD/CTRL/SAMPLE (standard/control/sample) on the protocol sheet. |
|
3. |
Take microtiter strips out of the aluminum bag. Store unused strips with desiccant at +4°C (+2-8°C) in the aluminum bag. Strips are stable until the expiry date stated on the label. |
|
4. |
Pipette 150 µl ASYBUF (assay buffer, red cap) into each well. |
|
4. |
Add 30 µl STD/CTRL/SAMPLE in dublicates into the respective wells. |
|
5. |
Add 50 µl CONJ (conjugate, amber cap) into each well. Swirl gently. |
|
6. |
Cover the plate tightly and incubate overnight (16-25 h) at +4°C (+2-8°C) in the dark. |
|
7. |
Aspirate and wash wells 5x with 300 µl diluted WASHBUF (wash buffer). After the final wash, remove remaining WASHBUF by strongly tapping plate against a paper towel. |
|
8. |
Add 200 µl SUB (substrate, blue cap) into each well. |
|
9. |
Incubate for 20 min at room temperature in the dark. |
|
10. |
Add 50 µl STOP (stop solution, white cap) into each well. |
|
11. |
Measure absorbance immediately at 450 nm with reference 630 nm, if available. |
Calculation of Results
Read the optical density (OD) of all wells on a plate reader using 450 nm wavelength (correction wavelength 630 nm). Construct a standard curve from the absorbance read-outs of the standards using commercially available software capable of generating a four-parameter logistic (4-PL) fit. Alternatively, plot the standards’ concentration on the x-axis against the mean absorbance for each standard on the y-axis and draw a best fit curve through the points on the graph. Curve fitting algorithms other than 4-PL have not been validated and will need to be evaluated by the user. Obtain sample concentrations from the standard curve. Respective dilution factors have to be considered when calculating the final concentration of the sample.
The quality control (QC) protocol supplied with the kit shows the results of the final release QC for each lot at production date. ODs obtained by customers may differ due to various influences including the normal decrease of signal intensity throughout shelf life. However, this does not affect validity of results as long as an OD of 1.5 or higher is obtained for STD 1 and the value of the CTRL is within the target range (see label).
BNP Fragment Protein
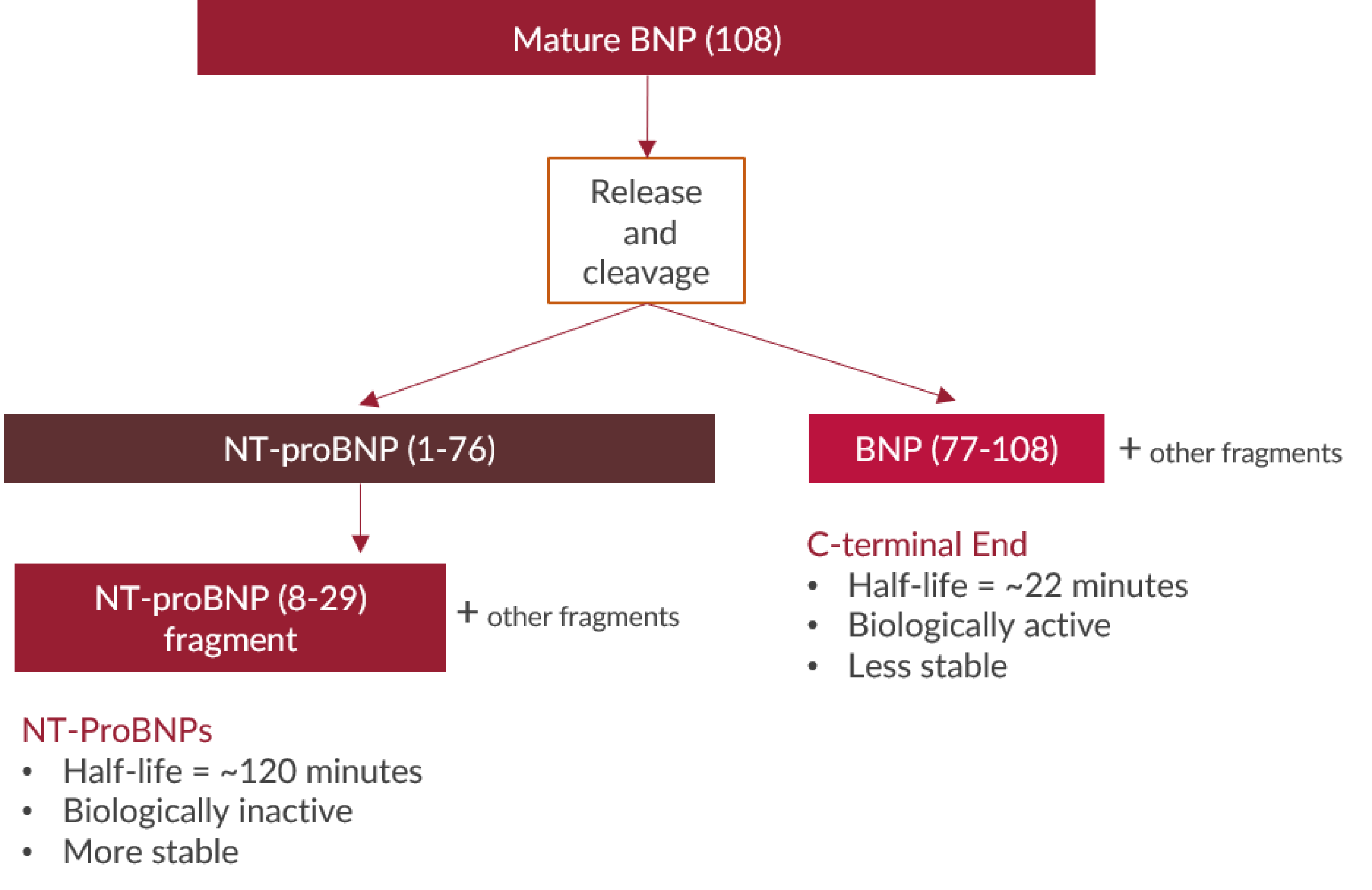
The natriuretic peptides are members of a family of structurally similar but genetically distinct peptide hormones, consisting of atrial-, brain-, and C-type (ANP, BNP, and CNP, respectively). ANP and BNP preferentially bind to a membrane-bound guanylyl cyclase (GC) receptor called GC-A or NPR1, whereas CNP is the physiological ligand for GC-B (NPR2). The natriuretic peptides play an important role in the regulation of cardiovascular and renal homeostasis and in the regulation of fatty acid metabolism and body weight.
BNP is mainly expressed by ventricular myocardium in response to volume overload and increased filling pressure. BNP has a cleavable signal sequence. Mature BNP consists of 108 amino acids (proBNP or BNP-108), and undergoes cleavage resulting in physiologically active BNP-32 and additional C-terminal fragments (cf. http://www.uniprot.org/uniprot/P16860#PRO_0000001532), along with a physiologically inactive N-terminal peptide comprising amino acids 1-76 (NT-proBNP), which is further degraded proteolytically.
From Alpco website https://www.alpco.com/physiological-actions-of-natriuretic-peptides
|
Molecular weight |
8.475 kDa |
|
Cellular localisation |
Secreted |
|
Post-translational modifications |
Glycosylation, disulfide bond |
|
Sequence similarities |
Natriuretic peptide family |
|
Alternative names |
BNP, Natriuretic Peptide B, NPPB, BNPT, |
|
Pubchem ID |
4879 link: https://www.ncbi.nlm.nih.gov/gene/4879 |
|
Genecards |
NPPB link: https://www.genecards.org/cgi-bin/carddisp.pl?gene=NPPB&keywords=nppb |
|
PDB |
1YK1: https://www.ebi.ac.uk/pdbe/entry/pdb/1YK1 |
|
OMIM |
600295 link: https://www.omim.org/entry/600295?search=nppb&highlight=nppb |
|
Protein Atlas |
NPPC link: https://www.proteinatlas.org/ENSG00000120937-NPPB/tissue |
|
Uniprot ID |
P16960 link: https://www.uniprot.org/uniprot/P16860 |
BNP Fragment Function
BNP has a key role in cardiovascular homeostasis with biological actions including natriuresis, diuresis, vasorelaxation, and inhibition of renin and aldosterone secretion. A high concentration of BNP in the bloodstream is indicative of heart failure.
The discovery of natriuretic peptides identified an endocrine system that contributes to diuresis and vascular tone. The biology, biochemistry and the pathophysiological role of natriuretic peptides are described in several reviews.
-
Heart & Cardiovascular disease
-
Acute myocardial infarction
-
Cardiac impairment
-
Risk assessment of rheumatoid arthritis patients under nonsteroidal anti-inflammatory agents/analgesics (NSAIAs)
-
Congenital heart disease
-
Coronary artery disease
-
Heart failure
-
Secondary hypertension
-
-
Nephrology
-
Renal disease
-
All Biomedica immunoassays are validated according to FDA/ICH/EMEA guidelines. For more information about our validation guidelines, please refer to our quality page and published validation guidelines and literature.
-
ICH Q2(R1) Validation of Analytical Procedures: Text and Methodology.
-
EMEA/CHMP/EWP/192217/2009 Guideline on bioanalytical method validation.
-
Bioanalytical Method Validation, Guidance for Industry, FDA, May 2018
Calibration
This NT-proBNP test kit is calibrated against recombinant NT-proBNP peptide.
NT-proBNP Test Kit Detection Limit & Sensitivity
To determine the sensitivity of the human total BNP fragment EIA, experiments measuring the lower limit of detection (LOD) were conducted.
|
LOD |
3.0 pmol/l |
NT-proBNP Test Kit Precision
The precision of an immunoassay is defined as its ability to measure the same concentration consistently within the same experiments carried out by one operator (within-run precision or repeatability) and across several experiments using the same samples but conducted by several operators at different locations using different ELISA lots (in-between-run precision or reproducibility).
Within-Run Precision
Two samples of known concentration were tested three times to assess within-run precision, also called intra-assay precision. The experiment was performed by one operator using a single plate.
|
ID |
n |
Mean BNP Fragment [pmol/l] |
SD [pmol/l] |
CV [%] |
|
Sample 1 |
3 |
763 |
43 |
6 |
|
Sample 2 |
3 |
3236 |
251 |
8 |
In-Between-Run Precision
Two samples of known concentration were tested eight times to assess in-between-run precision, also called inter-assay precision. The experiment was performed by different operators using plates and kit components from two lots.
|
ID |
n |
Mean BNP Fragment [pmol/l] |
SD [pmol/l] |
CV [%] |
|
Sample 1 |
8 |
781 |
45 |
6 |
|
Sample 2 |
8 |
3199 |
236 |
6 |
NT-proBNP Test Kit Accuracy
The accuracy of an immunoassay is defined as the precision with which it can recover samples of known concentrations.
The recovery of the BNP fragment EIA was measured by adding recombinant NT-proBNP to human samples containing a known concentration endogenous BNP fragment. The % recovery of the spiked concentration was calculated as the percentage of measured compared over the expected value. All our ELISAs are expected to have % recovery rates within 25% of the nominal value of the sample.
This table shows the summary of the recovery experiments in the BNP fragment human EIA in different sample matrices:
|
% Recovery |
|||||||
|
Sample Matrix |
n |
+ 533 pmol/l |
+ 2 133 pmol/l |
+ 4 889 pmol/l |
|||
|
Mean |
Range |
Mean |
Range |
Mean |
Range |
||
|
Serum |
4 |
107 |
84 - 125 |
94 |
78 - 113 |
||
|
Citrate plasma |
9 |
108 |
90 - 118 |
||||
Data showing recovery of BNP fragment in human serum samples:
|
BNP fragment [pmol/l] |
% Recovery |
|||||
|
Sample Matrix |
ID |
Reference |
+ 533 pmol/l |
+ 2 133 pmol/l |
+ 533 pmol/l |
+ 2 133 pmol/l |
|
Serum |
s1 |
1 241 |
1 709 |
3 128 |
96 |
93 |
|
Serum |
s2 |
2 069 |
2 194 |
3 258 |
84 |
78 |
|
Serum |
s3 |
66 |
746 |
2 091 |
125 |
95 |
|
Serum |
s4 |
0 |
645 |
2 403 |
121 |
113 |
|
Mean |
107 |
94 |
||||
|
Min |
84 |
78 |
||||
|
Max |
125 |
113 |
||||
Data showing recovery of BNP fragment in human citrate plasma samples:
|
BNP fragment [pmol/l] |
% Recovery |
|||
|
Sample Matrix |
ID |
Reference |
+ 4 889 pmol/l |
+ 4 889 pmol/l |
|
Citrate plasma |
c1 |
221 |
4,816 |
95 |
|
Citrate plasma |
c2 |
528 |
6,061 |
112 |
|
Citrate plasma |
c3 |
312 |
6,057 |
117 |
|
Citrate plasma |
c4 |
512 |
6,052 |
112 |
|
Citrate plasma |
c5 |
462 |
5,911 |
111 |
|
Citrate plasma |
c6 |
448 |
5,613 |
106 |
|
Citrate plasma |
c7 |
285 |
5,768 |
112 |
|
Citrate plasma |
c8 |
485 |
6,339 |
118 |
|
Citrate plasma |
c9 |
1,021 |
5,319 |
90% |
|
Mean |
108 |
|||
|
Min |
90 |
|||
|
Max |
118 |
|||
NT-proBNP Test Kit Dilution Linearity
Tests of dilution linearity and parallelism ensure that both endogenous and recombinant samples containing NT-proBNP (and fragments of NT-proBNP containing AA sequence 8-29) behave in a dose dependent manner and are not affected by matrix effects. Dilution linearity assesses the accuracy of measurements in diluted human samples spiked with known concentrations of recombinant analyte.
The table below shows the mean recovery and range of serially diluted recombinant BNP fragment in serum:
|
|
% Recovery of recombinant BNP fragment in diluted samples |
|||||
|
Sample Matrix |
n |
1+1 |
1+3 |
|||
|
Mean |
Range |
Mean |
Range |
|||
|
Serum |
7 |
105 |
94 – 113 |
131 |
98 - 154 |
|
Data showing dilution linearity of recombinant BNP fragment in human serum samples:
|
BNP fragment [pmol/l] |
% Recovery |
|||||
|
Sample matrix |
ID |
Reference |
1+1 |
1+3 |
1+1 |
1+3 |
|
Serum |
s1 |
1,384 |
661 |
661 |
96 |
98 |
|
Serum |
s2 |
2,388 |
1,236 |
1,236 |
104 |
131 |
|
Serum |
s3 |
2,214 |
1,216 |
1,216 |
110 |
141 |
|
Serum |
s4 |
3,387 |
1,588 |
1,588 |
94 |
114 |
|
Serum |
s5 |
2,642 |
1,464 |
1,464 |
111 |
154 |
|
Serum |
s6 |
2,482 |
1,360 |
1,360 |
110 |
143 |
|
Serum |
s7 |
2,451 |
1,382 |
1,382 |
113 |
137 |
|
Mean |
105 |
131 |
||||
|
Min |
94 |
98 |
||||
|
Max |
98 |
154 |
||||
NT-proBNP Test Kit Specificity
The BNP fragment EIA utilizes one antibody that binds to the amino acid region of 8-29 of the NT-proBNP peptide. Thus, the BNP fragment immunoassay is for the quantitative determination of NT-proBNP (1-76) (natural and recombinant), including all NT-proBNP derived fragments containing the amino acid sequence between AA 8-29.
Sample Stability
Sample Preparation
BNP fragments are stable in whole blood, serum or plasma for several hours at room temperature or +4°C (2-8°C). Nevertheless, we recommend separating plasma or serum by centrifugation as soon as possible, e.g. 20 min at 2,000 x g, preferably at +4°C (2-8°C). Aliquot the acquired plasma or serum samples and store them at -25°C or lower. Samples can be subjected to five freeze-thaw cycles without any loss of immune reactivity. Serum samples can be stored for ≥ 2 years at -80°C.
Overnight Storage
BNP fragment concentrations of samples after overnight storage at room temperature:
|
BNP fragment [pmol/l] |
% Recovery |
||
|
Sample ID |
Reference |
O.N. at RT |
|
|
#S1 |
803 |
732 |
91 |
|
#S2 |
1 247 |
1 392 |
112 |
|
#S3 |
2 480 |
2 477 |
100 |
|
Mean |
101 |
||
The recovery of human serum samples after an overnight storage at room temperature is 101%.
Sample Values
BNP Fragment Values in Apparently Healthy Donors
Total BNP fragment reference ranges were established using 76 serum samples from apparently healthy donors. No medical histories were available for the volunteers.
|
Sample Matrix |
n |
Median [pmol/l] |
Range [pmol/l] |
|
Serum |
76 |
392 |
150 – 1 631 |
We recommended establishing the normal range for each laboratory.
|
Sample ID |
BNP fragment [pmol/l] |
Sample ID |
BNP fragment [pmol/l] |
Sample ID |
BNP fragment [pmol/l] |
|
#1 |
166 |
#26 |
492 |
#51 |
359 |
|
#2 |
408 |
#27 |
420 |
#52 |
192 |
|
#3 |
576 |
#28 |
416 |
#53 |
341 |
|
#4 |
516 |
#29 |
614 |
#54 |
548 |
|
#5 |
351 |
#30 |
271 |
#55 |
342 |
|
#6 |
548 |
#31 |
335 |
#56 |
244 |
|
#7 |
348 |
#32 |
327 |
#57 |
398 |
|
#8 |
348 |
#33 |
428 |
#58 |
317 |
|
#9 |
250 |
#34 |
421 |
#59 |
253 |
|
#10 |
267 |
#35 |
598 |
#60 |
467 |
|
#11 |
270 |
#36 |
424 |
#61 |
611 |
|
#12 |
250 |
#37 |
337 |
#62 |
769 |
|
#13 |
895 |
#38 |
1,172 |
#63 |
269 |
|
#14 |
789 |
#39 |
348 |
#64 |
243 |
|
#15 |
407 |
#40 |
383 |
#65 |
150 |
|
#16 |
270 |
#41 |
321 |
#66 |
300 |
|
#17 |
162 |
#42 |
415 |
#67 |
244 |
|
#18 |
341 |
#43 |
523 |
#68 |
1,631 |
|
#19 |
383 |
#44 |
431 |
#69 |
431 |
|
#20 |
265 |
#45 |
453 |
#70 |
462 |
|
#21 |
364 |
#46 |
1,102 |
#71 |
784 |
|
#22 |
704 |
#47 |
455 |
#72 |
503 |
|
#23 |
243 |
#48 |
321 |
#73 |
1 045 |
|
#24 |
627 |
#49 |
578 |
#74 |
313 |
|
#25 |
287 |
#50 |
385 |
#75 |
629 |
|
#76 |
606 |
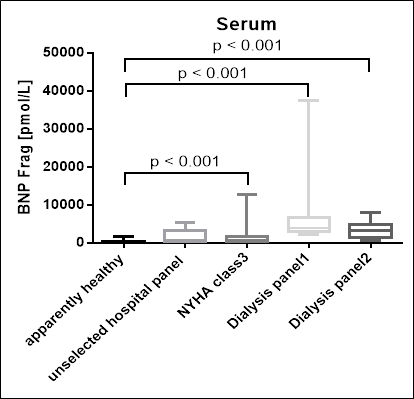
BNP Fragment Values in Two Dialysis and a Heart Failure Panel
BNP fragment was measured in serum samples of patients from two dialysis, an NYHA class 3 (heart failure) and an unselected hospital panel:
|
Cohort |
Sample Matrix |
n |
Median BNP fragment [pmol/l] |
Range [pmol/l] |
|
Dialysis panel 1 |
Serum |
16 |
3,426 |
2,280 – 3 426 |
|
Dialysis panel 2 |
Serum |
16 |
3,348 |
698 - >6 400 |
|
NYHA class 3 |
Serum |
95 |
775 |
49 – 12 800 |
|
Unselected hospital panel |
Serum |
32 |
574 |
171 – 5 438 |
The figure below shows the comparison of concentrations of BNP fragment in several patient panels with apparently healthy donors:
Concentrations of 16 human serum samples from a dialysis panel 1 derived from a hospital lab:
-
Sample ID
BNP fragment [pmol/l]
#1
3 200
#2
6 081
#3
2 280
#4
>6 400
#5
3 070
#6
>6 400
#7
4 178
#8
>6 400
#9
3 863
#10
4 036
#11
2 806
#12
4 142
#13
3 653
#14
2 374
#15
2 893
#16
>6 400
Concentrations of 16 human serum samples from a dialysis panel 2 derived from a hospital lab:
-
Sample ID
BNP fragment [pmol/l]
#1
3 375
#2
6 120
#3
>6 400
#4
1 197
#5
4 331
#6
5 146
#7
781
#8
698
#9
3 321
#10
>6 400
#11
1 386
#12
3 549
#13
4 370
#14
2 662
#15
2 072
#16
1 404
Matrix Comparison
Correlation of Serum and Plasma Samples from Apparently Healthy Individuals
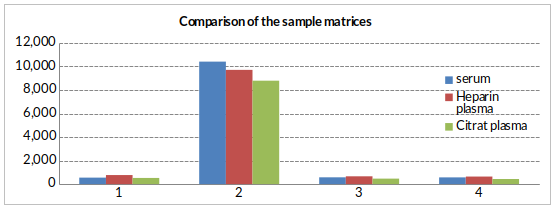
Measurement of BNP Fragment in 3 different sample matrices from 4 samples showed a mean CV of 17%. All 3 matrices can be tested in the assay.
Data showing the effect of the sample matrix:
|
BNP fragment [pmol/l] |
|||||
|
Sample ID |
Serum |
Heparın plasma |
Citrate plasma |
Mean |
CV |
|
#1 |
545 |
770 |
523 |
613 |
22% |
|
#2 |
10,408 |
9,708 |
8,792 |
9,636 |
8% |
|
#3 |
577 |
664 |
462 |
567 |
18% |
|
#4 |
568 |
640 |
435 |
547 |
19% |
|
Mean |
17% |
||||
Figure showing matrix comparison of BNP fragment sample concentrations between serum, heparın plasma, and citrate plasma in an apparently healthy cohort (n=4).
- Myocarditis in Cynomolgus Monkeys Following Treatment with Immune Checkpoint Inhibitors.
Ji, C., Roy, M.D., Golas, J., Vitsky, A., Ram, S., Kumpf, S.W., Martin, M., Barletta, F., Meier, W., Hooper, A.T., Sapra, P., Khan, N.K., Finkelstein, M., Guffroy, M., Buetow, B.S., 2019. Clin Cancer Res clincanres.4083.2018.
PMID:31085720
- P1563Reducing late maternal death due to cardiovascular disease by targeted interventions.
Azibani, F., Libhaber, E., Baard, J., Osman, A., Zuhlke, L., Lachmann, A., Chin, A., Ntsekhe, M., Soma-Pillay, P., Johnson, M.R., Roos-Hesselink, J., Anthony, J., Sliwa, K., 2018, in: European Heart Journal.
- P057 Effects of ANTI-TNF therapy on vascular biomarker levels in rheumatoid arthritis.
Hamar, A., Végh, E., Horváth, Á., Szántó, S., Szucs, G., Pusztai, A., Domján, A., Hodosi, K., Kerekes, G., Gesztelyi, R., Zsuga, J., Prohászka, Z., Szekanecz, Z., 2018, in: Annals of the Rheumatic Diseases. pp. A37–A37.
- Does Ramadan Fasting Affect Hydration Status and Kidney Function in CKD Patients?
Hassan, S., Hassan, F., Abbas, N., Hassan, K., Khatib, N., Edgim, R., Fadol, R., Khazim, K., 2018. ANM 72, 241–247.
PMID:29518785
- P058 S100 proteins effectively discriminate systemic lupus erythematosus patients from healthy controls, but are not associated with measures of disease activity.
Šumová, B., Závada, J., Cerezo, L.A., Uher, M., Hulejová, H., Grigorian, M., Pavelka, K., Vencovský, J., Šenolt, L., 2018. Annals of the Rheumatic Diseases 77, A37–A38.
- Correlation between APOE gene polymorphisms and efficacy of trimetazidine in treating chronic heart failure secondary to non-ischemic cardiomyopathy: a population-based study in China.
Sun, S., Yang, Y., Pei, H., 2018 11
- FRI0057 Effects of anti-tnf therapy on vascular biomarker levels in rheumatoid arthritis.
Végh, E., Hamar, A., Horváth, Á., Szántó, S., Szücs, G., Pusztai, A., Domján, A., Hodosi, K., Kerekes, G., Gesztelyi, R., Zsuga, J., Prohászka, Z., Szekanecz, Z., 2018, in: Annals of the Rheumatic Diseases. pp. 575–576.
- SERAPHIN haemodynamic substudy: the effect of the dual endothelin receptor antagonist macitentan on haemodynamic parameters and NT-proBNP levels and their association with disease progression in patients with pulmonary arterial hypertension.
Galiè, N., Jansa, P., Pulido, T., Channick, R.N., Delcroix, M., Ghofrani, H.-A., Le Brun, F.-O., Mehta, S., Perchenet, L., Rubin, L.J., Sastry, B.K.S., Simonneau, G., Sitbon, O., Souza, R., Torbicki, A., 2017. Eur. Heart J. 38, 1147–1155. PMCID: PMC5400052
PMID:28329315
- Prognostic Value of Left Ventricular Diastolic Dysfunction in a General Population.
Kuznetsova, T., Thijs, L., Knez, J., Herbots, L., Zhang, Z., Staessen, J.A., 2014. J Am Heart Assoc 3. PMCID: PMC4309065
PMID:24780207
- Parathyroid hormone is associated with the LV mass after aortic valve replacement.
Laflamme, M.-H., Mahjoub, H., Mahmut, A., Boulanger, M.-C., Larose, E., Pibarot, P., Mathieu, P., 2014. Heart 100, 1859–1864.
PMID:25095827
- Function of N-terminal pro-brain natriuretic peptide in Takayasu arteritis disease monitoring.
Liu, Q., Dang, A., Chen, B., Lv, N., Wang, X., Zheng, D., 2014. J. Rheumatol. 41, 1683–1688.
PMID:25028376
- Predicting the risk of venous thromboembolism in patients hospitalized with heart failure.
Mebazaa, A., Spiro, T.E., Büller, H.R., Haskell, L., Hu, D., Hull, R., Merli, G., Schellong, S.W., Spyropoulos, A.C., Tapson, V.F., De Sanctis, Y., Cohen, A.T., 2014. Circulation 130, 410–418.
PMID:24970782
- Spectrum of cardiac disease in maternity in a low-resource cohort in South Africa.
Sliwa, K., Libhaber, E., Elliott, C., Momberg, Z., Osman, A., Zühlke, L., Lachmann, T., Nicholson, L., Thienemann, F., Roos-Hesselink, J., Anthony, J., 2014. Heart 100, 1967–1974. PMCID: PMC4251204
PMID:25227705
- Brain natriuretic peptide predicts functional outcome in ischemic stroke.
Rost, N.S., Biffi, A., Cloonan, L., Chorba, J., Kelly, P., Greer, D., Ellinor, P., Furie, K.L., 2012. Stroke 43, 441–445. PMCID: PMC3265658
PMID:22116811
- Multidimensional assessment of older people with asthma and COPD: clinical management and health status.
McDonald, V.M., Simpson, J.L., Higgins, I., Gibson, P.G., 2011. Age Ageing 40, 42–49.
PMID:21087988
- Increased expression of growth differentiation factor-15 in systemic sclerosis-associated pulmonary arterial hypertension.
Meadows, C.A., Risbano, M.G., Zhang, L., Geraci, M.W., Tuder, R.M., Collier, D.H., Bull, T.M., 2011. Chest 139, 994–1002. PMCID: PMC3087455
PMID:20829333
- Characterization of molecular forms of N-terminal B-type natriuretic peptide in vitro.
Ala-Kopsala, M., Moilanen, A.-M., Rysä, J., Ruskoaho, H., Vuolteenaho, O., 2010. Clin. Chem. 56, 1822–1829.
PMID:20926601
- Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction.
González, A., López, B., Querejeta, R., Zubillaga, E., Echeverría, T., Díez, J., 2010. Hypertension 55, 1418–1424.
PMID:20404218
- Myocardial gene expression alterations in peripheral blood mononuclear cells of patients with idiopathic dilated cardiomyopathy.
Kontaraki, J.E., Parthenakis, F.I., Nyktari, E.G., Patrianakos, A.P., Vardas, P.E., 2010. European Journal of Heart Failure 12, 541–548.
- Comparison of Pleural Fluid N-Terminal Pro-Brain Natriuretic Peptide and Brain Natriuretic-32 Peptide Levels.
Long, A.C., O’Neal, H.R., Peng, S., Lane, K.B., Light, R.W., 2010. Chest 137, 1369–1374. 20139229
PMID:20139229
- Clinical value of a competitive NT-proBNP enzyme immunoassay compared to the Roche NT-proBNP platform.
Hammerer-Lercher, A., Griesmacher, A., Pölzl, G., Brinskelle-Schmal, N., Mair, J., Frick, M., Hawa, G., 2009. Clin. Chem. Lab. Med. 47, 1305–1308.
PMID:19751139
- Effect of piboserod, a 5-HT4 serotonin receptor antagonist, on left ventricular function in patients with symptomatic heart failure.
Kjekshus, J.K., Torp-Pedersen, C., Gullestad, L., Køber, L., Edvardsen, T., Olsen, I.C., Sjaastad, I., Qvigstad, E., Skomedal, T., Osnes, J.-B., Levy, F.O., 2009. Eur. J. Heart Fail. 11, 771–778.
PMID:19567409
- Prevalence of left ventricular diastolic dysfunction in a general population.
Kuznetsova, T., Herbots, L., López, B., Jin, Y., Richart, T., Thijs, L., González, A., Herregods, M.-C., Fagard, R.H., Díez, J., Staessen, J.A., 2009. Circ Heart Fail 2, 105–112.
PMID:19808325
- Asymmetrical dimethylarginine in systemic sclerosis-related pulmonary arterial hypertension.
Dimitroulas, T., Giannakoulas, G., Sfetsios, T., Karvounis, H., Dimitroula, H., Koliakos, G., Settas, L., 2008. Rheumatology (Oxford) 47, 1682–1685.
PMID:18753191
- N-Terminal-ProB-Type Natriuretic Peptide as a Marker for Anthracycline Cardiotoxicity in Children with Acute Lymphoblastic Leukaemia.
Jackowska, T., Golabek, M., 2008. Blood 112, 3965–3965
- NT-proBNP response to dobutamine stress echocardiography predicts left ventricular contractile reserve in dilated cardiomyopathy.
Parthenakis, F.I., Patrianakos, A.P., Haritakis, C.N., Zacharis, E.A., Nyktari, E.G., Vardas, P.E., 2008. Eur. J. Heart Fail. 10, 475–481.
PMID:18396456
- Immunodetection of glycosylated NT-proBNP circulating in human blood.
Seferian, K.R., Tamm, N.N., Semenov, A.G., Tolstaya, A.A., Koshkina, E.V., Krasnoselsky, M.I., Postnikov, A.B., Serebryanaya, D.V., Apple, F.S., Murakami, M.M., Katrukha, A.G., 2008. Clin. Chem. 54, 866–873.
PMID:18339697
- NT-proBNP levels and diastolic dysfunction in beta-thalassaemia major patients.
Kremastinos, D.T., Tsiapras, D.P., Kostopoulou, A.G., Hamodraka, E.S., Chaidaroglou, A.S., Kapsali, E.D., 2007. Eur. J. Heart Fail. 9, 531–536.
PMID:17317307
- Effects of nocturnal noninvasive mechanical ventilation on heart rate variability of patients with advanced COPD.
Sin, D.D., Wong, E., Mayers, I., Lien, D.C., Feeny, D., Cheung, H., Gan, W.Q., Man, S.F.P., 2007. Chest 131, 156–163.
PMID:17218570
- Correlation between serial measurements of N-terminal pro brain natriuretic peptide and ambulatory cardiac filling pressures in outpatients with chronic heart failure.
Braunschweig, F., Fahrleitner-Pammer, A., Mangiavacchi, M., Ghio, S., Fotuhi, P., Hoppe, U.C., Linde, C., 2006. European Journal of Heart Failure 8, 797–803.
- Reduced apelin levels in lone atrial fibrillation.
Ellinor, P.T., Low, A.F., Macrae, C.A., 2006. Eur. Heart J. 27, 222–226.
PMID:16278229
- Coronary and peripheral blood flow changes following biventricular pacing and their relation to heart failure improvement.
Flevari, P., Theodorakis, G., Paraskevaidis, I., Kolokathis, F., Kostopoulou, A., Leftheriotis, D., Kroupis, C., Livanis, E., Kremastinos, D.T., 2006. Europace 8, 44–50.
PMID:16627408
- The effect of thyroid dysfunction on N-terminal pro-B-type natriuretic peptide concentrations.
Manuchehri, A.M., Jayagopal, V., Kilpatrick, E.S., Atkin, S.L., 2006. Ann. Clin. Biochem. 43, 184–188.
PMID:16704752
- N-terminal brain natriuretic peptide as a screening tool for heart failure in the pacemaker population.
Thackray, S.D.R., Witte, K., Ghosh, J., Nikitin, N., Anderson, A., Rigby, A., Goode, K., Clark, A.L., Cleland, J.G.F., 2006. Eur. Heart J. 27, 447–453.
PMID:16299020
- Neurohormonal risk stratification for sudden death and death owing to progressive heart failure in chronic heart failure.
Berger, R., Huelsmann, M., Strecker, K., Moertl, D., Moser, P., Bojic, A., Pacher, R., 2005. Eur. J. Clin. Invest. 35, 24–31.
PMID:15638816
- Prognostic value of plasma N-terminal pro-brain natriuretic peptide in patients with severe sepsis.
Brueckmann, M., Huhle, G., Lang, S., Haase, K.K., Bertsch, T., Weiss, C., Kaden, J.J., Putensen, C., Borggrefe, M., Hoffmann, U., 2005. Circulation 112, 527–534.
PMID:16027260
- Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation.
Ellinor, P.T., Low, A.F., Patton, K.K., Shea, M.A., Macrae, C.A., 2005. J. Am. Coll. Cardiol. 45, 82–86.
PMID:15629379
- N-Terminal Pro-B-Type Natriuretic Peptide as an Indicator of Possible Cardiovascular Disease in Severely Obese Individuals: Comparison with Patients in Different Stages of Heart Failure.
Hermann-Arnhof, K.-M., Hanusch-Enserer, U., Kaestenbauer, T., Publig, T., Dunky, A., Rosen, H.R., Prager, R., Köller, U., 2005. Clinical Chemistry 51, 138–143.
PMID:15550477
- Risk assessment in patients with unstable angina/non-ST-elevation myocardial infarction and normal N-terminal pro-brain natriuretic peptide levels by N-terminal pro-atrial natriuretic peptide.
Jarai, Rudolf, Iordanova, N., Jarai, Robert, Raffetseder, A., Woloszczuk, W., Gyöngyösi, M., Geyer, G., Wojta, J., Huber, K., 2005. Eur. Heart J. 26, 250–256.
PMID:15618049
- Is the pregnancy hormone relaxin an important player in human heart failure?
Kupari, M., Mikkola, T.S., Turto, H., Lommi, J., 2005. Eur. J. Heart Fail. 7, 195–198.
PMID:15701466
- Natriuretic Peptides as Markers of Mild Forms of Left Ventricular Dysfunction: Effects of Assays on Diagnostic Performance of Markers.
Hammerer-Lercher, A., Ludwig, W., Falkensammer, G., Müller, S., Neubauer, E., Puschendorf, B., Pachinger, O., Mair, J., 2004. Clinical Chemistry 50, 1174–1183.
PMID:15142976
- Elevated plasma levels of Nt-proBNP in patients with type 2 diabetes without overt cardiovascular disease.
Magnusson, M., Melander, O., Israelsson, B., Grubb, A., Groop, L., Jovinge, S., 2004. Diabetes Care 27, 1929–1935
PMID:15277419
- Atrial and brain natriuretic peptides as markers of response to resynchronisation therapy.
Molhoek, S.G., Bax, J.J., van Erven, L., Bootsma, M., Steendijk, P., Lentjes, E., Boersma, E., van der Laarse, A., van der Wall, E.E., Schalij, M.J., 2004. Heart 90, 97–98. PMCID: PMC1768004
PMID:14676258
- Restrictive filling pattern is associated with increased humoral activation and impaired exercise capacity in dilated cardiomyopathy.
Patrianakos, A.P., Parthenakis, F.I., Papadimitriou, E.A., Diakakis, G.F., Tzerakis, P.G., Nikitovic, D., Vardas, P.E., 2004. Eur. J. Heart Fail. 6, 735–743.
PMID:15542409
- Plasma concentrations of aminoterminal pro atrial natriuretic peptide and aminoterminal pro brain natriuretic peptide in healthy neonates: marked and rapid increase after birth.
Mir, T.S., Laux, R., Hellwege, H.H., Liedke, B., Heinze, C., von Buelow, H., Läer, S., Weil, J., 2003. Pediatrics 112, 896–899
PMID:14523183
- Comparison of the Biomedica NT-proBNP enzyme immunoassay and the Roche NT-proBNP chemiluminescence immunoassay: implications for the prediction of symptomatic and asymptomatic structural heart disease.
Mueller, T., Gegenhuber, A., Poelz, W., Haltmayer, M., 2003. Clin. Chem. 49, 976–979
PMID:12766002
- B-type natriuretic peptide predicts sudden death in patients with chronic heart failure.
Berger, R., Huelsman, M., Strecker, K., Bojic, A., Moser, P., Stanek, B., Pacher, R., 2002. Circulation 105, 2392–2397
PMID:12021226
- Prediction of outcome by neurohumoral activation, the six-minute walk test and the Minnesota Living with Heart Failure Questionnaire in an outpatient cohort with congestive heart failure.
Hülsmann, M., Berger, R., Sturm, B., Bojic, A., Woloszczuk, W., Bergler-Klein, J., Pacher, R., 2002. Eur. Heart J. 23, 886–891.
PMID:12042010
- Plasma concentrations of N-terminal pro-brain natriuretic peptide in control children from the neonatal to adolescent period and in children with congestive heart failure.
Mir, T.S., Marohn, S., Läer, S., Eiselt, M., Grollmus, O., Weil, J., 2002. Pediatrics 110, e76
PMID:12456943
- Clinical relevance of cardiac natriuretic peptides measured by means of competitive and non-competitive immunoassay methods in patients with renal failure on chronic hemodialysis.
Clerico, A., Caprioli, R., Del Ry, S., Giannessi, D., 2001. J. Endocrinol. Invest. 24, 24–30.
PMID:11227728
- Early Post-Operative Haemodynamic and Neurohumoral Follow-Up After Endoaneurysmorrhaphy.
Jain, D., Grimm, M., Bartels, C., Bechtel, M., Tölg, R., Hartmann, F., Katus, H.A., Sievers, H.H., Richardt, G., 2001 4
- Measurement and importance of plasma brain natriuretic peptide and related peptides.
Sagnella, G.A., 2001. Annals of Clinical Biochemistry 38, 83–93.
- Prognostic evaluation of neurohumoral plasma levels before and during beta-blocker therapy in advanced left ventricular dysfunction.
Stanek, B., Frey, B., Hülsmann, M., Berger, R., Sturm, B., Strametz-Juranek, J., Bergler-Klein, J., Moser, P., Bojic, A., Hartter, E., Pacher, R., 2001. J. Am. Coll. Cardiol. 38, 436–442
PMID:11499735
- Measurement of cardiac natriuretic hormones (atrial natriuretic peptide, brain natriuretic peptide, and related peptides) in clinical practice: the need for a new generation of immunoassay methods.
Clerico, A., Del Ry, S., Giannessi, D., 2000. Clin. Chem. 46, 1529–1534
PMID:11017928
 Download biomedica product list
Download biomedica product list