FluoBolt™ Klotho FIA | FIA-1704
-
Method
Metal Enhanced Direct Sandwich Fluorescence Immunoassay in 96-well plate format
-
Sample type
Serum, Plasma
-
Sample volume
10 µl / well
-
Assay time
overnight / room temperature (18 – 26°C)
-
Sensitivity
LOD (0 pmol/l + 3 SD): 2,5 pmol/l; LLOQ: 25 pmol/l
-
Standard range
0 – 400 pmol/l (= 0 – 24,840 pg/ml)
-
Conversion factor
1 ng/ml = 16 pmol/l (MW: 62.1 kDa)
-
Cross-reactivity
Human KLOTHO shares around 98 97% aa sequence with higher apes, 95 91% bovines, 91 89% pinnipeds and 87% mice. Cross reactivity of this assay with other species than human has not been tested.
-
Detection target
This assay specifically detects human endogenous and recombinant α KLOTHO. No cross-reactivity with β KLOTHO.
-
Regulatory status
Research use only
-
Detection range
2.5 – 400 pmol/l (= 155 – 24,840 pg/ml)
-
Precision
In-between-run (n=4): ≤ 10 % CV
Within-run (n=4): ≤ 11 % CV
Product Overview
The FluoBolt™-KLOTHO immunoassay is an o.n. Metal Enhanced Direct Sandwich Fluorescence Immunoassay in 96-well plate format for the quantitative determination of KLOTHO in serum and plasma. The assay employs human based serum standards to ensure the measurement of biologically reliable data.
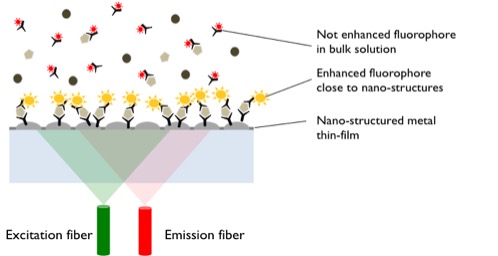
Principle Of The Assay
The FluoBolt™-KLOTHO immunoassay is an o.n. Metal Enhanced Direct Sandwich Fluorescence Immunoassay in 96-well plate format for the quantitative determination of KLOTHO in serum and plasma samples.
Figure explaining the principle of metal enhanced fluorescence:
In a first step, standard/sample/control and detection antibody (fluorescent labeled anti-KLOTHO) are pipetted into the wells of the microtiter plate, which are pre-coated with anti-KLOTHO antibody. KLOTHO present in the standard/sample/control binds to the pre-coated antibody in the well and forms a sandwich with the detection antibody.
The signal of the bound fluorescent detection antibody is enhanced several hundred fold by the metal nano-structures at the plate bottom and thus highly sensitive detectable with a standard microtiter plate fluorescence reader. Measurements can either been done without washing (bottom measurement) or after a final washing step (top measurement). The concentration of KLOTHO in the sample is determined directly from the dose response curve.
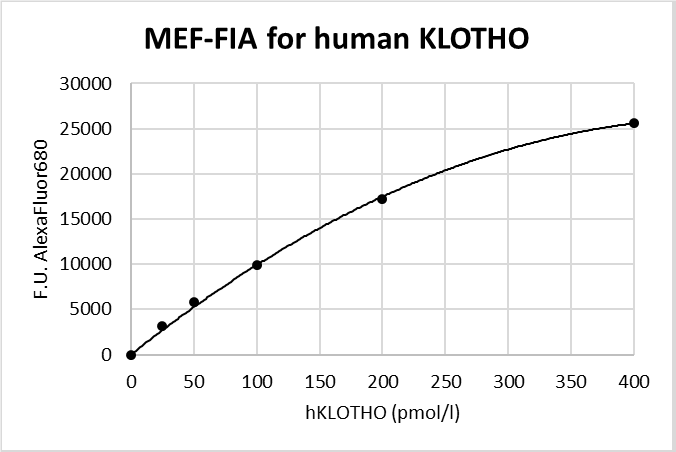
Typical Standard Curve
The figure below shows a typical standard curve for the FluoBolt™-KLOTHO ELISA. The immunoassay is calibrated against recombinant full length KLOTHO:
Kit Components
|
Contents |
Description |
Quantity |
|
HM |
Anti human KLOTHO antibody, pre-coated MEF microtiter plate, packed in vacuum sealed aluminum bag |
1 x 96 well |
|
WP |
20X wash buffer concentrate |
1 x 25 ml |
|
HAF, HA3, HA5, HAA |
Anti human KLOTHO antibody, black flask, labeled with FITC, Cy3, Cy5 or AlexaFluor 680 |
1 x 5 ml |
|
HS |
Standards 1-6, (400, 200, 100, 50, 25, 0 pmol/l), white caps, lyophilized |
6 vials, 0.25 ml |
Storage instructions: All reagents of the FluoBolt™-KLOTHO immunoassay kit are stable at 4°C until the expiry date stated on the label of each reagent.
Sample Collection & Storage
Collect venous blood samples by using standardized blood collection tubes for serum or plasma. We recommend performing plasma or serum separation by centrifugation as soon as possible, e.g. 10 min at 2000 x g, preferably at 4°C (2-8°C). The acquired plasma or serum samples should be measured as soon as possible. For longer storage aliquot samples and store at -25°C or lower. Do not freeze-thaw samples more than 4 times.
Lipemic or hemolyzed samples may give erroneous results. Samples should be mixed well before assaying.
Reagent Preparation
Add 250 μl of distilled or deionized water to the lyophilized HS (Standards) and HC (Controls). Leave at room temperature (18 26°C) for 15 min. Reconstituted HS and HC are stable at 25°C or lower until expiry date stated on the label. Reconstituted HS and HC can undergo 4 freeze thaw cycles.
Bring WP (Wash buffer) concentrate (20x) to room temperature. Make sure that the solution is clear and without any salt precipitates before further dilution. Dilute the WP to working strength by adding the appropriate amount of distilled or deionized water, e.g. 25 ml of WP + 475 ml water, prior to use in the assay. Undiluted WP is stable at 4°C (2
8°C) until expiry date on the label. Diluted WP is stable at 4°C (2 8°C) up to one month. Only use diluted WP in the assay.
Sample Preparation
All reagents and samples must be at room temperature (18-26°C) before use in the assay.
Assay Protocol
Read the entire protocol before beginning the assay.
In standard format, the kit is delivered with an Alexa Fluor 680 labeled detection antibody (DAA) because serum background fluorescence is minimal within this wavelength range. Therefore if your reader is equipped with monochromatic optics, please set Excitation/Emission to 679/702 nm or if you are using an optical filter based reader, select a suitable filter pair (e.g. 670/720 nm). On request the kit can also be delivered with FITC, Cy3 or Cy5 (Ex/Em = 495/518 nm, 550/570 nm or 650/670 nm) labeled detection antibody
Mark positions for DS/ Sample/ DC (Standard/ Sample/ Control) on the protocol sheet.
|
1. |
Take the plasmonic enhanced microtiter plate out of the aluminum bag. Avoid touching the bottom of the plate with bare hands, because reading without washing is performed through the well bottom. |
|
2. |
Add 50 µl of the selected fluorescence labeled detection antibody (HAF or HA3 or HA5 or HAA) to all wells required. Swirl gently. |
|
3. |
Add 10 µl of standard, control or sample to the wells according to the marked positions on the protocol sheet, swirl gently. |
|
4. |
Cover tightly with the delivered adhesive film and incubate over night at 37°C in the dark. |
|
5a. |
If your reader allows bottom reading, read the plate without any further processing at the Ex/Em wavelength fitting to the delivered detection antibody (495/518 nm for HAF, 550/570 nm for HA3, 650/670 nm for HA5, 679/702 nm for HAA). Gain should be set to achieve at least 10000 fluorescence units (F.U.) between the signal of the 0 pmol/l and the 400 pmol/l KLOTHO standard. Samples with signals exceeding the signal of the highest standard must be re run with an appropriate dilution using sample diluent (HD). |
|
5b. |
If your reader has no bottom read option or if you want to store the plate for documentation purposes, discard or aspirate the content of the wells and wash 3x with diluted wash buffer. Use a minimum of 200 µl wash buffer per well. After the final wash, remove remaining fluid by strongly tapping the plate against a paper towel. Read the plate in top configuration without any further processing at the Ex/Em wavelength fitting to the chosen detection antibody (495/518 nm for HAF, 550/570 nm for HA3, 650/670 nm for HA5, 679/702 nm for HAA). |
|
! |
Hint: Quality of bottom reading (5a) may vary between microplate readers. For first time users we suggest to perform the washing step and follow protocol 5b. |
|
6. |
Gain should be set to achieve at least 10000 fluorescence units (F.U.) between the signals of the 0 pmol/l and the 400 pmol/l KLOTHO standard. Samples with signals exceeding the signal of the highest standard must be re run with appropriate dilution using sample diluent (HD). |
|
7. |
Store the plate with the 2 desiccant bags supplied at 4°C (2 8°C) in the aluminum bag. Unused wells are stable until expiry date stated on the label. Fluorescence signals of standards, controls and samples remain detectable for at least two months at the plate surface, depending on signal intensity achieved. |
Calculation Of Results
Subtract the fluorescence intensity of the 0 pmol/l standard from all other standards, samples and controls. Construct a calibration curve from the fluorescence units (F.U.) of the standards using commercially available software or graph paper. Read sample and control concentrations from this standard curve. Make sure to use appropriate curve fitting algorithm (e.g. linear or 4PL).
The quality control (QC) protocol supplied with the kit shows the results of the final release QC for each kit lot at production date.
Fluorescence intensity obtained by customers may differ due to various influences and/or due to the normal decrease of signal intensity during shelf life. However, this does not affect validity of results as long as the supplied kit controls read according to specifications (target ranges see labels).
INFORMATION ON THE ANALYTE
α KLOTHO is a protein primarily expressed in kidney. It can be found either as a membrane bound or a secreted form. The membrane bound form consists of 1012 amino acids (aa), starting with a 56 aa long signalling sequence and followed by two glycosyl hydrolase 1 regions (position 57 506 and 515 953). Both glycosyl hydrolase 1 regions lack one essential Glu active site residue. Thus, it is inactive in vivo as a glycosidase although it belongs to the glycosyl hydrolase 1 family. KLOTHO’s secreted isoform, which predominates over the membrane bound form, consists of 549 amino acids (aa). It is produced by alternative splicing and differs from the membrane bound form by aa 550 to 1012 missing.
|
Molecular Weight |
116,181 (whole molecule) |
|
Cellular localisation |
membrane and secreted |
|
Post-translational modifications |
N-glycosylated |
|
Sequence similarities |
Glycoside Hydrolase Family 1 |
|
Alternative Names |
none |
|
Entrez/NCBI ID |
9365 |
|
Genecards |
KL |
|
OMIM |
604824 |
|
PDB |
5W21 |
|
Pfam |
PF00232 |
|
Protein Atlas |
Q9UEF7 |
|
Uniport ID |
Q9UEF7 |
FUNCTION
α KLOTHO is expressed in kidney, small intestine, placenta and prostate. The soluble peptide can be found in serum and cerebrospinal fluid. It may play a role in the calcium/ phosphorus homeostasis regulation by e.g. inhibiting active vitamin D synthesis. Further, it is also known as an anti
aging hormone by extending life span by inhibiting insulin/ IGF1 signalling pathway, as experiments in mice showed. KLOTHO is a co receptor of fibroblast growth factor 23 (FGF 23). Research has investigated association of altered serum KLOTHO levels with chronic kidney disease and failure, renal and hepatocellular carcinomas, osteoporosis or cardiovascular diseases.
Literature
Single step, direct fluorescence immunoassays based on metal enhanced fluorescence (MEF-FIA) applicable as micro plate-, array-, multiplexing- or point of care-format. Hawa G et al., Anal Biochem. 2018;549:39-44.
The Prognostic Role of Klotho in Patients with Chronic Kidney Disease: A Systematic Review and Meta-analysis. Liu QF, Yu LX, Feng JH, Sun Q, Li SS, Ye JM. Dis Markers. 2019 Jun; 2019:6468729.
Klotho plays a critical role in clear cell renal cell carcinoma progression and clinical outcome. Kim JH, Hwang KH, Lkhagvadorj S, Jung JH, Chung HC, Park KS, Kong ID, Eom M, Cha SK. Korean J Physiol Pharmacol. 2016 May; 20(3):297-304.
The Biological Role of Klotho Protein in the Development of Cardiovascular Diseases. Olejnik A, Franczak A, Krzywonos-Zawadzka A, Kałużna-Oleksy M, Bil-Lula I. Biomed Res Int. 2018 Dec; 2018:5171945.
Klotho and chronic kidney disease. Hu MC, Kuro-o M, Moe OW. Contrib Nephrol. 2013; 180:47-63.
Pathophysiological implications of fibroblast growth factor-23 and Klotho and their potential role as clinical biomarkers. Donate-Correa J, Muros de Fuentes M, Mora-Fernández C, Navarro-González JF. Clin Chem. 2014 Jul; 60(7):933-40.
-
Cardiology
Cardiovascular diseases
-
Nephrology
Chronic Kidney Disease (CKD)
-
Oncology
Renal- and Hepatocellular Carcinomas
All FluoBolt™ IMMUNOASSAYs are validated according to sensitivity, specificity, precision, accuracy, dilution linearity, sample stability, expected values in blood donor collections and sample matrix .
Calibration
The FluoBolt™-KLOTHO immunoassay is calibrated against recombinant human KLOTHO protein.
Detection Limit & Sensitivity
To determine the sensitivity of the FluoBolt™-KLOTHO immunoassay, experiments measuring the Lower Limit of Detection (LOD) and the lower limit of quantification (LLOQ) were conducted.
The LOD, also called the detection limit, is the lowest point at which a signal can be distinguished above the background signal, i.e. the signal that is measured in the absence of KLOTHO, with a confidence level of 99%. It is defined as the mean back calculated concentration of standard 1 (0 pmol/l of KLOTHO, three independent measurements) plus three times the standard deviation of the measurements.
The LLOQ, or sensitivity of an assay, is the lowest concentration at which an analyte can be accurately quantified. The criteria for accurate quantification at the LLOQ are an analyte recovery between 75 and 125% and a coefficient of variation (CV) of less than 25%. To determine the LLOQ, standard 2, i.e. the lowest standards containing KLOTHO, is diluted, measured three times and its concentration back calculated. The lowest dilution, which meets both criteria, is reported as the LLOQ.
The following values were determined for the FluoBolt™-KLOTHO immunoassay:
|
LOD |
2.5 pmol/l |
|
LLOQ |
25 pmol/l |
Precision
The precision of an FluoBolt™-KLOTHO immunoassay is defined as its ability to measure the same concentration consistently within the same experiments carried out by one operator (within-run precision or repeatability) and across several experiments using the same samples but conducted by several operators at different locations using different FluoBoltTM KLOTHO immunoassay lots (in-between-run precision or reproducibility).
Within-Run Precision
Within-run precision was tested by measuring the same 4 samples 3 times within one FluoBolt™-KLOTHO immunoassay lot. The experiment was conducted by one operator. Samples reading above the highest standard were diluted with the sample diluent provided in the kit. CVs ranged from 1 - 11.0%
In-Between-Run Precision
In-between-run precision was assessed by measuring the same 4 samples 3 times within multiple FluoBolt™-KLOTHO immunoassay lots. The measurements were carried out by one operator. CVs ranged from 6 - 10%.
Accuracy
The accuracy of an FluoBolt™-KLOTHO immunoassay is defined as the precision with which it can recover samples of known concentrations.
The recovery of KLOTHO in serum was evaluated by adding known amounts of human recombinant KLOTHO to 4 different human serum samples. Mean recovery was 82% (69 101%).
Dilution Linearity
Tests of dilution linearity and parallelism ensure that both endogenous and recombinant samples containing KLOTHO behave in a dose dependent manner and are not affected by matrix effects. Dilution linearity assesses the accuracy of measurements in diluted clinical samples spiked with known concentrations of recombinant analyte.
3 human serum samples were spiked with recombinant KLOTHO and diluted 1+1 and 1+2 with the sample diluent (HD) supplied with the kit. Mean linearity was 69% (see table below).
-
Measured (pM)
Dilution
Sample #1
Sample #2
Sample #3
1+0
292
239
209
1+1
168
182
166
1+2
152
115
106
Expected (pM)
1+1
146
120
104
1+2
97
80
70
Linearity (%)
1+1
87%
66%
63%
1+2
64%
69%
66%
Specificity
Analyte Specificity:
This assay detects human α KLOTHO. Addition of recombinant FGF 23, which is considered to be binding to KLOTHO, to the standards supplied with this kit did not reduce signal intensity. Measurement of β KLOTHO is not possible with this kit. The presence of β KLOTHO does not interfere with the detection of α KLOTHO.
Species Specificity:
Human KLOTHO shares around 98 97% aa sequence with higher apes (e.g. orangutan or chimpanzee), 95 91% bovines (e.g. cattle or yak), 91 89% pinnipeds (e.g. walrus or monk seal) and 87% mice. Reactivity of this assay with other species than human has not been tested. So, using this assay for KLOTHO measurements in serum or plasma of species with high sequence homology may be possible but must be evaluated by the user. FIANOSTICS does not take responsibility for functionality of the assay in non human samples.
 Download biomedica product list
Download biomedica product list